Stages of benign thyroid nodules: principles and ultrasound signs
- PMID: 39143992
- PMCID: PMC11320486
- DOI: 10.21037/qims-24-477
Stages of benign thyroid nodules: principles and ultrasound signs
Abstract
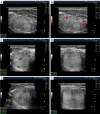
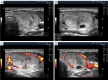
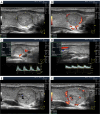
Benign thyroid nodules are significantly common and occur in 50-60% of the population. Therefore, differentiation from malignant nodes and the choice of treatment tactics in some cases of benign pathology remain relevant. Despite advances in the clinical evaluation of thyroid nodules, methodological challenges exist due to empirically simplistic understandings of the nodular process. Different opinions on the pathogenesis of thyroid nodules and the history of the formation of the idea of the stages of nodules are considered. For the first time, based on natural principles and many years of ultrasound analysis of changes in benign thyroid nodules, three stages of the nodular process were identified: Development, Wasting and Scarring. The stage of exhaustion has three substages: Initial, Moderate and Significant Wasting. The principles of stage-by-stage changes in nodules are explained and their ultrasound signs are shown. The key principle of the stages of nodules is the ratio of the magnitudes of the processes of regeneration (proliferation) and destruction in the nodule. Separate stage changes may occur in node segments. In such cases, part of the segments may show signs of the Development stage, another part-Wasting, and the third part-Scarring. The different variants of thyroid nodules are explained in terms of stages. Practical recommendations for differentiating ultrasound signs of nodules associated with stages are proposed. Knowledge about the staged changes in thyroid nodules helps reduce the likelihood of diagnostic errors, better navigate the prognosis and choice of treatment tactics, and recommend preventive ultrasound examination of the thyroid.
Keywords: Thyroid nodules; nodules stage; thyroid ultrasound.
2024 Quantitative Imaging in Medicine and Surgery. All rights reserved.
Conflict of interest statement
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-477/coif). The author has no conflicts of interest to declare.
Figures

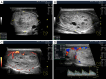
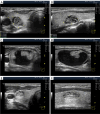
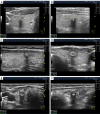
References
-
- Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedüs L, Paschke R, Valcavi R, Vitti P, AACE/ACE/AME Task Force on Thyroid Nodules . American association of clinical endocrinologists, American college of endocrinology, and associazione medici endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules--2016 update. Endocr Pract 2016;22:622-39. 10.4158/EP161208.GL - DOI - PubMed
-
- Grani G, Bruno R, Lucisano G, Costante G, Meringolo D, Puxeddu E, Torlontano M, Tumino S, Attard M, Lamartina L, Nicolucci A, Cooper DS, Filetti S, Durante C. Temporal Changes in Thyroid Nodule Volume: Lack of Effect on Paranodular Thyroid Tissue Volume. Thyroid 2017;27:1378-84. 10.1089/thy.2017.0201 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
