Unpacking the packaged optical fiber bio-sensors: understanding the obstacle for biomedical application
- PMID: 39144482
- PMCID: PMC11322460
- DOI: 10.3389/fbioe.2024.1401613
Unpacking the packaged optical fiber bio-sensors: understanding the obstacle for biomedical application
Abstract
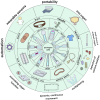
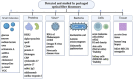
A biosensor is a promising alternative tool for the detection of clinically relevant analytes. Optical fiber as a transducer element in biosensors offers low cost, biocompatibility, and lack of electromagnetic interference. Moreover, due to the miniature size of optical fibers, they have the potential to be used in microfluidic chips and in vivo applications. The number of optical fiber biosensors are extensively growing: they have been developed to detect different analytes ranging from small molecules to whole cells. Yet the widespread applications of optical fiber biosensor have been hindered; one of the reasons is the lack of suitable packaging for their real-life application. In order to translate optical fiber biosensors into clinical practice, a proper embedding of biosensors into medical devices or portable chips is often required. A proper packaging approach is frequently as challenging as the sensor architecture itself. Therefore, this review aims to give an unpack different aspects of the integration of optical fiber biosensors into packaging platforms to bring them closer to actual clinical use. Particularly, the paper discusses how optical fiber sensors are integrated into flow cells, organized into microfluidic chips, inserted into catheters, or otherwise encased in medical devices to meet requirements of the prospective applications.
Keywords: biosensor; microfluidic; optical fiber; packaged; wearable.
Copyright © 2024 Bissen, Yunussova, Myrkhiyeva, Salken, Tosi and Bekmurzayeva.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




References
-
- Abdul Hamid I. S. L., Mustapha Kamil Y., Abd Manaf A., Mahdi M. A. (2017). Fabrication and characterization of micro fluidic based fiber optic refractive index sensor. Sens. Biosensing Res. 13, 70–74. 10.1016/j.sbsr.2017.03.003 - DOI
-
- Afsarimanesh N., Nag A., Sarkar S., Sabet G. S., Han T., Mukhopadhyay S. C. (2020). A review on fabrication, characterization and implementation of wearable strain sensors. Sens. Actuators A Phys. 315, 112355. 10.1016/j.sna.2020.112355 - DOI
-
- Arasu P. T., Noor A. S. M., Shabaneh A. A., Yaacob M. H., Lim H. N., Mahdi M. A. (2016). Fiber Bragg grating assisted surface plasmon resonance sensor with graphene oxide sensing layer. Opt. Commun. 380, 260–266. 10.1016/j.optcom.2016.05.081 - DOI
Publication types
LinkOut - more resources
Full Text Sources

