Tape-assisted fabrication method for constructing PDMS membrane-containing culture devices with cyclic radial stretching stimulation
- PMID: 39144495
- PMCID: PMC11321861
- DOI: 10.1098/rsos.240284
Tape-assisted fabrication method for constructing PDMS membrane-containing culture devices with cyclic radial stretching stimulation
Abstract
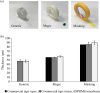
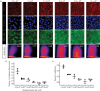
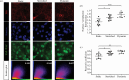
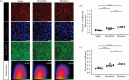
Advanced in vitro culture systems have emerged as alternatives to animal testing and traditional cell culture methods in biomedical research. Polydimethylsiloxane (PDMS) is frequently used in creating sophisticated culture devices owing to its elastomeric properties, which allow mechanical stretching to simulate physiological movements in cell experiments. We introduce a straightforward method that uses three types of commercial tape-generic, magic and masking-to fabricate PDMS membranes with microscale thicknesses (47.2 µm for generic, 58.1 µm for magic and 89.37 µm for masking) in these devices. These membranes are shaped as the bases of culture wells and can perform cyclic radial movements controlled via a vacuum system. In experiments with A549 cells under three mechanical stimulation conditions, we analysed transcriptional regulators responsive to external mechanical stimuli. Results indicated increased nuclear yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) activity in both confluent and densely packed cells under cyclically mechanical strains (Pearson's coefficient (PC) of 0.59 in confluent and 0.24 in dense cells) compared with static (PC = 0.47 in confluent and 0.13 in dense) and stretched conditions (PC = 0.55 in confluent and 0.20 in dense). This technique offers laboratories without microfabrication capabilities a viable option for exploring cellular behaviour under dynamic mechanical stimulation using PDMS membrane-equipped devices.
Keywords: PDMS membrane; YAP/TAZ; microfabrication; microphysiological systems.
© 2024 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures




Similar articles
-
Different in vitro cellular responses to tamoxifen treatment in polydimethylsiloxane-based devices compared to normal cell culture.J Chromatogr B Analyt Technol Biomed Life Sci. 2017 Nov 15;1068-1069:105-111. doi: 10.1016/j.jchromb.2017.09.041. Epub 2017 Oct 12. J Chromatogr B Analyt Technol Biomed Life Sci. 2017. PMID: 29073477
-
Floating-on-water Fabrication Method for Thin Polydimethylsiloxane Membranes.Polymers (Basel). 2019 Jul 31;11(8):1264. doi: 10.3390/polym11081264. Polymers (Basel). 2019. PMID: 31370158 Free PMC article.
-
Type I collagen deposition via osteoinduction ameliorates YAP/TAZ activity in 3D floating culture clumps of mesenchymal stem cell/extracellular matrix complexes.Stem Cell Res Ther. 2018 Dec 7;9(1):342. doi: 10.1186/s13287-018-1085-9. Stem Cell Res Ther. 2018. PMID: 30526677 Free PMC article.
-
Beyond Polydimethylsiloxane: Alternative Materials for Fabrication of Organ-on-a-Chip Devices and Microphysiological Systems.ACS Biomater Sci Eng. 2021 Jul 12;7(7):2880-2899. doi: 10.1021/acsbiomaterials.0c00640. Epub 2020 Sep 9. ACS Biomater Sci Eng. 2021. PMID: 34275293 Review.
-
Yes-associated protein (YAP) and transcriptional coactivator with a PDZ-binding motif (TAZ): a nexus between hypoxia and cancer.Acta Pharm Sin B. 2020 Jun;10(6):947-960. doi: 10.1016/j.apsb.2019.12.010. Epub 2019 Dec 19. Acta Pharm Sin B. 2020. PMID: 32642404 Free PMC article. Review.
References
-
- Mirbagheri M, Adibnia V, Hughes BR, Waldman SD, Banquy X, Hwang DK. 2019. Advanced cell culture platforms: a growing quest for emulating natural tissues. Mater. Horiz. 6 , 45–71. (10.1039/C8MH00803E) - DOI
-
- Leung CM, et al. . 2022. A guide to the organ-on-a-chip. Nat. Rev. Methods Primers 2 , 33. (10.1038/s43586-022-00118-6) - DOI
Associated data
LinkOut - more resources
Full Text Sources

