Recent Advances in Photoinduced Oxidative Cleavage of Alkenes
- PMID: 39144683
- PMCID: PMC11323056
- DOI: 10.1055/s-0042-1751534
Recent Advances in Photoinduced Oxidative Cleavage of Alkenes
Abstract
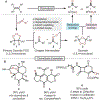
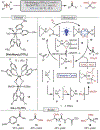
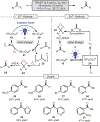
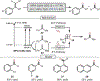
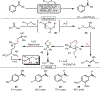
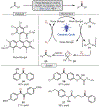
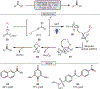
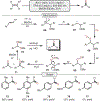
Oxidative cleavage of alkenes leading to valuable carbonyl derivatives is a fundamental transformation in synthetic chemistry. In particular, ozonolysis is the mainstream method for the oxidative cleavage of alkenes that has been widely implemented in the synthesis of natural products and pharmaceutically relevant compounds. However, due to the toxicity and explosive nature of ozone, alternative approaches employing transition metals and enzymes in the presence of oxygen and/or strong oxidants have been developed. These protocols are often conducted under harsh reaction conditions that limit the substrate scope. Photochemical approaches can provide milder and more practical alternatives for this synthetically useful transformation. In this review, we outline recent visible-light-promoted oxidative cleavage reactions that involve photocatalytic activation of oxygen via electron transfer and energy transfer. Also, an emerging field featuring visible-light-promoted oxidative cleavage under anaerobic conditions is discussed. The methods highlighted in this review represent a transformative step toward more sustainable and efficient strategies for the oxidative cleavage of alkenes.
Keywords: aerobic; alkenes; anaerobic; nitroarenes; oxidative cleavage; ozonolysis; photochemistry; visible light.
Conflict of interest statement
Conflict of Interest The authors declare no conflict of interest.
Figures
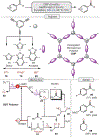
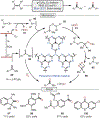


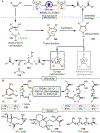
References
-
- Modern Carbonyl Chemistry; Otera J, Ed.; Wiley-VCH: Wein-heim, 2000.
-
- Rubin MB Bull. Hist. Chem 2001, 26, 40.
-
- Schönbein CF Ann. Phys. (Berlin, Ger.) 1840, 126, 616.
-
- Schönbein CF Br. Assoc. Adv. Sci., Rep 1840, 209; available at www.biodiversitylibrary.org.
-
- Harries CD Justus Liebigs Ann. Chem 1905, 343, 311.
Grants and funding
LinkOut - more resources
Full Text Sources
