The amorphization of crystalline silicon by ball milling
- PMID: 39144970
- PMCID: PMC11320443
- DOI: 10.1016/j.heliyon.2024.e34881
The amorphization of crystalline silicon by ball milling
Abstract
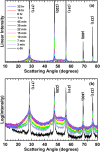
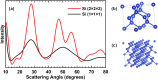
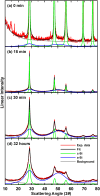
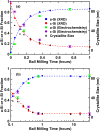
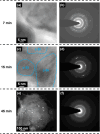
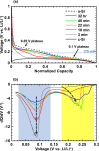
The transformation of crystalline silicon to amorphous silicon during ball milling was quantitatively measured by x-ray diffraction and electrochemical methods. Amorphous silicon was found to form rapidly from the very initial stages of ball milling. Simultaneously, the grain size of the crystalline silicon phase decreased. Under extended milling times it was found that a maximum of 86 % of the silicon became amorphous. Similarly, the grain size of the crystalline silicon phase could not be reduced below 6 nm. This transformation followed an Avrami kinetic model, which is consistent with a system which reaches a steady state. These observations suggest a mechanism in which ball milling generates defects, resulting in silicon amorphization and grain size reduction, where the degree of amorphization is limited in extent because there exists a limiting silicon grain size below which defects are no longer formed.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Obrovac M.N. Si-alloy negative electrodes for Li-ion batteries. Curr. Opin. Electrochem. 2018;9:8–17. doi: 10.1016/j.coelec.2018.02.002. - DOI
-
- Obrovac M.N. Lithium-Ion Batteries Enabled by Silicon Anodes. Institution of Engineering and Technology; 2021. Overview of the development of silicon anodes for lithium-ion batteries; pp. 1–50. - DOI
-
- Ulvestad A., Reksten A.H., Andersen H.F., Carvalho P.A., Jensen I.J.T., Nagell M.U., Mæhlen J.P., Kirkengen M., Koposov A.Y. Crystallinity of silicon nanoparticles: direct influence on the electrochemical performance of lithium ion battery anodes. Chemelectrochem. 2020;7:4349–4353. doi: 10.1002/celc.202001108. - DOI
LinkOut - more resources
Full Text Sources

