Unveiling the shield: Troglitazone's impact on epilepsy-induced nerve injury through ferroptosis inhibition
- PMID: 39145422
- PMCID: PMC11325165
- DOI: 10.1111/cns.14911
Unveiling the shield: Troglitazone's impact on epilepsy-induced nerve injury through ferroptosis inhibition
Abstract
Background: Epilepsy is a widespread central nervous system disorder with an estimated 50 million people affected globally. It is characterized by a bimodal incidence peak among infants and the elderly and is influenced by a variety of risk factors, including a significant genetic component. Despite the use of anti-epileptic drugs (AEDs), drug-refractory epilepsy develops in about one-third of patients, highlighting the need for alternative therapeutic approaches.
Aims: The primary aim of this study was to evaluate the neuroprotective effects of troglitazone (TGZ) in epilepsy and to explore the potential mechanisms underlying its action.
Methods: We employed both in vitro and in vivo models to assess TGZ's effects. The in vitro model involved glutamate-induced toxicity in HT22 mouse hippocampal neurons, while the in vivo model used kainic acid (KA) to induce epilepsy in mice. A range of methods, including Hoechst/PI staining, CCK-8 assay, flow cytometry, RT-PCR analysis, Nissl staining, scanning electron microscopy, and RNA sequencing, were utilized to assess various parameters such as cellular damage, viability, lipid-ROS levels, mitochondrial membrane potential, mRNA expression, seizure grade, and mitochondrial morphology.
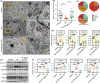
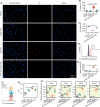
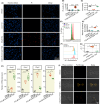
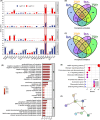
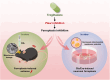
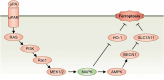
Results: Our results indicate that TGZ, at doses of 5 or 20 mg/kg/day, significantly reduces KA-induced seizures and neuronal damage in mice by inhibiting the process of ferroptosis. Furthermore, TGZ was found to prevent changes in mitochondrial morphology. In the glutamate-induced HT22 cell damage model, 2.5 μM TGZ effectively suppressed neuronal ferroptosis, as shown by a reduction in lipid-ROS accumulation, a decrease in mitochondrial membrane potential, and an increase in PTGS2 expression. The anti-ferroptotic effect of TGZ was confirmed in an erastin-induced HT22 cell damage model as well. Additionally, TGZ reversed the upregulation of Plaur expression in HT22 cells treated with glutamate or erastin. The downregulation of Plaur expression was found to alleviate seizures and reduce neuronal damage in the mouse hippocampus.
Conclusion: This study demonstrates that troglitazone has significant therapeutic potential in the treatment of epilepsy by reducing epileptic seizures and the associated brain damage through the inhibition of neuronal ferroptosis. The downregulation of Plaur expression plays a crucial role in TGZ's anti-ferroptotic effect, offering a promising avenue for the development of new epilepsy treatments.
Keywords: Plaur; brain damage; epilepsy; ferroptosis; neuroprotection; troglitazone.
© 2024 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no potential conflict of interest.
Figures


Similar articles
-
JC124 confers multimodal neuroprotection in epilepsy by suppressing NLRP3 inflammasome activation: evidence from animal and human neuronal models.Cell Commun Signal. 2025 Jul 8;23(1):327. doi: 10.1186/s12964-025-02239-3. Cell Commun Signal. 2025. PMID: 40629339 Free PMC article.
-
Levetiracetam add-on for drug-resistant focal epilepsy: an updated Cochrane Review.Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD001901. doi: 10.1002/14651858.CD001901.pub2. Cochrane Database Syst Rev. 2012. PMID: 22972056 Free PMC article.
-
Exploring the Therapeutic Potential of Baicalin: Mitigating Anxiety and Depression in Epileptic Rats.Comb Chem High Throughput Screen. 2025;28(10):1776-1794. doi: 10.2174/0113862073316021240520110301. Comb Chem High Throughput Screen. 2025. PMID: 38778616
-
Rufinamide add-on therapy for refractory epilepsy.Cochrane Database Syst Rev. 2018 Apr 25;4(4):CD011772. doi: 10.1002/14651858.CD011772.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 Nov 8;11:CD011772. doi: 10.1002/14651858.CD011772.pub3. PMID: 29691835 Free PMC article. Updated.
-
GLS2 reduces the occurrence of epilepsy by affecting mitophagy function in mouse hippocampal neurons.CNS Neurosci Ther. 2024 Oct;30(10):e70036. doi: 10.1111/cns.70036. CNS Neurosci Ther. 2024. PMID: 39404053 Free PMC article.
References
-
- Ding D, Zhou D, Sander JW, Wang W, Li S, Hong Z. Epilepsy in China: major progress in the past two decades. Lancet Neurol. 2021;20(4):316‐326. - PubMed
-
- Mateen FJ. Treating epilepsy in forcibly displaced persons: timely, necessary, affordable. Nat Rev Neurol. 2021;17(10):593‐594. - PubMed
-
- González Stivala E, Sarudiansky M, Wolfzun C, et al. Comorbid impulsivity after one year of epilepsy surgery. Epilepsy Behav. 2021;124:108331. - PubMed
-
- Löscher W, Klein P. New approaches for developing multi‐targeted drug combinations for disease modification of complex brain disorders. Does epilepsy prevention become a realistic goal? Pharmacol Ther. 2022;229:107934. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2023M741145/China Postdoctoral Science Foundation
- kq2208155/Changsha Municipal Natural Science Foundation
- 2023JJ40415/Hunan Provincial Natural Science Foundation of China
- kh2003010/Major Science and Technology Program of Changsha
- 2021 JC0002/Major Project of Natural Science Foundation of Hunan Province
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

