MNK-driven eIF4E phosphorylation regulates the fibrogenic transformation of mesenchymal cells and chronic lung allograft dysfunction
- PMID: 39145446
- PMCID: PMC11324311
- DOI: 10.1172/JCI168393
MNK-driven eIF4E phosphorylation regulates the fibrogenic transformation of mesenchymal cells and chronic lung allograft dysfunction
Abstract
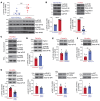
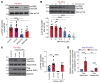
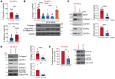
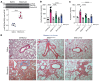
Tissue fibrosis remains unamenable to meaningful therapeutic interventions and is the primary cause of chronic graft failure after organ transplantation. Eukaryotic translation initiation factor (eIF4E), a key translational regulator, serves as convergent target of multiple upstream profibrotic signaling pathways that contribute to mesenchymal cell (MC) activation. Here, we investigate the role of MAP kinase-interacting serine/threonine kinase-induced (MNK-induced) direct phosphorylation of eIF4E at serine 209 (Ser209) in maintaining fibrotic transformation of MCs and determine the contribution of the MNK/eIF4E pathway to the pathogenesis of chronic lung allograft dysfunction (CLAD). MCs from patients with CLAD demonstrated constitutively higher eIF4E phosphorylation at Ser209, and eIF4E phospho-Ser209 was found to be critical in regulating key fibrogenic protein autotaxin, leading to sustained β-catenin activation and profibrotic functions of CLAD MCs. MNK1 signaling was upregulated in CLAD MCs, and genetic or pharmacologic targeting of MNK1 activity inhibited eIF4E phospho-Ser209 and profibrotic functions of CLAD MCs in vitro. Treatment with an MNK1/2 inhibitor (eFT-508) abrogated allograft fibrosis in an orthotopic murine lung-transplant model. Together these studies identify what we believe is a previously unrecognized MNK/eIF4E/ATX/β-catenin signaling pathway of fibrotic transformation of MCs and present the first evidence, to our knowledge, for the utility of MNK inhibitors in fibrosis.
Keywords: Cell migration/adhesion; Extracellular matrix; Pulmonology; Translation; Transplantation.
Conflict of interest statement
Figures



References
-
- Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-eighth adult lung transplantation report - 2021; Focus on recipient characteristics. J Heart Lung Transplant. 2021;40(10):1060–1072. doi: 10.1016/j.healun.2021.07.021. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

