Mouse sarcopenia model reveals sex- and age-specific differences in phenotypic and molecular characteristics
- PMID: 39145448
- PMCID: PMC11324300
- DOI: 10.1172/JCI172890
Mouse sarcopenia model reveals sex- and age-specific differences in phenotypic and molecular characteristics
Abstract
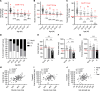
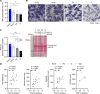
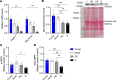
Our study was to characterize sarcopenia in C57BL/6J mice using a clinically relevant definition to investigate the underlying molecular mechanisms. Aged male (23-32 months old) and female (27-28 months old) C57BL/6J mice were classified as non-, probable-, or sarcopenic based on assessments of grip strength, muscle mass, and treadmill running time, using 2 SDs below the mean of their young counterparts as cutoff points. A 9%-22% prevalence of sarcopenia was identified in 23-26 month-old male mice, with more severe age-related declines in muscle function than mass. Females aged 27-28 months showed fewer sarcopenic but more probable cases compared with the males. As sarcopenia progressed, a decrease in muscle contractility and a trend toward lower type IIB fiber size were observed in males. Mitochondrial biogenesis, oxidative capacity, and AMPK-autophagy signaling decreased as sarcopenia progressed in males, with pathways linked to mitochondrial metabolism positively correlated with muscle mass. No age- or sarcopenia-related changes were observed in mitochondrial biogenesis, OXPHOS complexes, AMPK signaling, mitophagy, or atrogenes in females. Our results highlight the different trajectories of age-related declines in muscle mass and function, providing insights into sex-dependent molecular changes associated with sarcopenia progression, which may inform the future development of novel therapeutic interventions.
Keywords: Aging; Mitochondria; Mouse models; Muscle biology; Skeletal muscle.
Conflict of interest statement
Figures




Comment in
- Decoding the decline: unveiling drivers of sarcopenia doi: 10.1172/JCI183302
References
-
- Du K, et al. Prevalence of sarcopenia and sarcopenic obesity vary with race/ethnicity and advancing age. Divers Equal Health Care. 2018;15(4):175–183. doi: 10.21767/2049-5471.1000173. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

