Potassium-competitive Acid Blockers: Current Clinical Use and Future Developments
- PMID: 39145848
- PMCID: PMC11401795
- DOI: 10.1007/s11894-024-00939-3
Potassium-competitive Acid Blockers: Current Clinical Use and Future Developments
Abstract
Purpose of the review: Acid suppression with proton pump inhibitors (PPIs) represents the standard of care in the treatment of acid-related diseases. However, despite their effectiveness, PPIs display some intrinsic limitations, which underlie the unmet clinical needs that have been identified over the past decades. The aims of this review are to summarize the current status and future development of the new class of antisecretory drugs (potassium-competitive acid blockers, P-CABs) that have recently been introduced into medical practice.
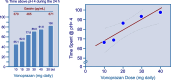
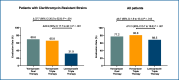
Recent findings: Over the past decades, clinical needs unmet by the current acid suppressants have been recognized, especially in the management of patients with GERD, Helicobacter pylori infection and NSAID-related peptic ulcer. The failure to address these needs is mainly due to their inability to achieve a consistent acid suppression in all patients and, particularly, to control nighttime acidity. It was then realized that an extended duration of acid suppression would exert additional benefits. The available data with P-CABs show that they are able to address these unmet clinical needs. Four different P-CABs (vonoprazan, tegoprazan, fexuprazan and keverprazan) are currently available. However, only two of them are approved outside Asia. Vonoprazan is available in North, Central and South America while tegoprazan is marketed only in Latin American countries. Two other compounds (namely linazapran glurate and zestaprazan) are presently under clinical development. While clinical trials on GERD have been performed with all P-CABs, only vonoprazan and tegoprazan have been investigated as components of Helicobacter pylori eradication regimens. The available data show that-in the above two clinical indications-P-CABs provide similar or better efficacy in comparison with PPIs. Their safety in the short-term overlaps that of PPIs, but data from long-term treatment are needed.
Keywords: H. pylori Infection; Fexuprazan; GERD; Kerverprazan; Linaprazan glurate; P-CABs; PPIs; Tegoprazan; Unmet Clinical Needs; Vonoprazan; Zestaprazan.
© 2024. The Author(s).
Conflict of interest statement
Professor Scarpignato is member of the Advisory Board of Phathom and Professor Hunt is member of the Advisory Board of Cinclus. The first Company developed and is marketing a P-CAB (vonoprazan) in the US, while the second one is developing another P-CAB (namely Linaprazan glurate).
Figures




References
-
- Savarino V, Di Mario F, Scarpignato C. Proton pump inhibitors in GORD: an overview of their pharmacology, efficacy and safety. Pharmacol Res. 2009;59(3):135–53. 10.1016/j.phrs.2008.09.016. - PubMed
-
- Scarpignato C, Gatta L. Acid suppression for management of gastroesophageal reflux disease: benefits and risks. In: Morice A, Dettmar P (eds) Reflux Aspiration and Lung Disease, Springer International Publishing AG, Cham 2018;269–291. 10.1007/978-3-319-90525-9_23.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

