Mechanism of gabapentinoid potentiation of opioid effects on cyclic AMP signaling in neuropathic pain
- PMID: 39145932
- PMCID: PMC11348325
- DOI: 10.1073/pnas.2405465121
Mechanism of gabapentinoid potentiation of opioid effects on cyclic AMP signaling in neuropathic pain
Abstract
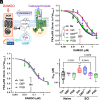
Over half of spinal cord injury (SCI) patients develop opioid-resistant chronic neuropathic pain. Safer alternatives to opioids for treatment of neuropathic pain are gabapentinoids (e.g., pregabalin and gabapentin). Clinically, gabapentinoids appear to amplify opioid effects, increasing analgesia and overdose-related adverse outcomes, but in vitro proof of this amplification and its mechanism are lacking. We previously showed that after SCI, sensitivity to opioids is reduced by fourfold to sixfold in rat sensory neurons. Here, we demonstrate that after injury, gabapentinoids restore normal sensitivity of opioid inhibition of cyclic AMP (cAMP) generation, while reducing nociceptor hyperexcitability by inhibiting voltage-gated calcium channels (VGCCs). Increasing intracellular Ca2+ or activation of L-type VGCCs (L-VGCCs) suffices to mimic SCI effects on opioid sensitivity, in a manner dependent on the activity of the Raf1 proto-oncogene, serine/threonine-protein kinase C-Raf, but independent of neuronal depolarization. Together, our results provide a mechanism for potentiation of opioid effects by gabapentinoids after injury, via reduction of calcium influx through L-VGCCs, and suggest that other inhibitors targeting these channels may similarly enhance opioid treatment of neuropathic pain.
Keywords: Cav1.2; adenylyl cyclase; dorsal root ganglia; opioid; pregabalin.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Gurba K. N., Chaudhry R., Haroutounian S., Central neuropathic pain syndromes: Current and emerging pharmacological strategies. CNS Drugs 36, 483–516 (2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

