Breast Cancer Index in Premenopausal Women With Early-Stage Hormone Receptor-Positive Breast Cancer
- PMID: 39145953
- PMCID: PMC11327904
- DOI: 10.1001/jamaoncol.2024.3044
Breast Cancer Index in Premenopausal Women With Early-Stage Hormone Receptor-Positive Breast Cancer
Abstract
Importance: Adjuvant ovarian function suppression (OFS) with oral endocrine therapy improves outcomes for premenopausal patients with hormone receptor-positive (HR+) breast cancer but adds adverse effects. A genomic biomarker for selecting patients most likely to benefit from OFS-based treatment is lacking.
Objective: To assess the predictive and prognostic performance of the Breast Cancer Index (BCI) for OFS benefit in premenopausal women with HR+ breast cancer.
Design, setting, and participants: This prospective-retrospective translational study used all available tumor tissue samples from female patients from the Suppression of Ovarian Function Trial (SOFT). These individuals were randomized to receive 5 years of adjuvant tamoxifen alone, tamoxifen plus OFS, or exemestane plus OFS. BCI testing was performed blinded to clinical data and outcome. The a priori hypothesis was that BCI HOXB13/IL17BR ratio (BCI[H/I])-high tumors would benefit more from OFS and high BCI portended poorer prognosis in this population. Settings spanned multiple centers internationally. Participants included premenopausal female patients with HR+ early breast cancer with specimens in the International Breast Cancer Study Group tumor repository available for RNA extraction. Data were collected from December 2003 to April 2021 and were analyzed from May 2022 to October 2022.
Main outcomes and measures: Primary end points were breast cancer-free interval (BCFI) for the predictive analysis and distant recurrence-free interval (DRFI) for the prognostic analyses.
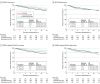
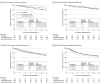
Results: Tumor specimens were available for 1718 of the 3047 female patients in the SOFT intention-to-treat population. The 1687 patients (98.2%) who had specimens that yielded sufficient RNA for BCI testing represented the parent trial population. The median (IQR) follow-up time was 12 (10.5-13.4) years, and 512 patients (30.3%) were younger than 40 years. Tumors were BCI(H/I)-low for 972 patients (57.6%) and BCI(H/I)-high for 715 patients (42.4%). Patients with tumors classified as BCI(H/I)-low exhibited a 12-year absolute benefit in BCFI of 11.6% from exemestane plus OFS (hazard ratio [HR], 0.48 [95% CI, 0.33-0.71]) and an absolute benefit of 7.3% from tamoxifen plus OFS (HR, 0.69 [95% CI, 0.48-0.97]) relative to tamoxifen alone. In contrast, patients with BCI(H/I)-high tumors did not benefit from either exemestane plus OFS (absolute benefit, -0.4%; HR, 1.03 [95% CI, 0.70-1.53]; P for interaction = .006) or tamoxifen plus OFS (absolute benefit, -1.2%; HR, 1.05 [95% CI, 0.72-1.54]; P for interaction = .11) compared with tamoxifen alone. BCI continuous index was significantly prognostic in the N0 subgroup for DRFI (n = 1110; P = .004), with 12-year DRFI of 95.9%, 90.8%, and 86.3% in BCI low-risk, intermediate-risk, and high-risk N0 cancers, respectively.
Conclusions and relevance: In this prospective-retrospective translational study of patients enrolled in SOFT, BCI was confirmed as prognostic in premenopausal women with HR+ breast cancer. The benefit from OFS-containing adjuvant endocrine therapy was greater for patients with BCI(H/I)-low tumors than BCI(H/I)-high tumors. BCI(H/I)-low status may identify premenopausal patients who are likely to benefit from this more intensive endocrine therapy.
Conflict of interest statement
Figures


References
-
- Davies C, Godwin J, Gray R, et al. ; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771-784. doi: 10.1016/S0140-6736(11)60993-8 - DOI - PMC - PubMed
-
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: a patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol. 2022;23(3):382-392. doi: 10.1016/S1470-2045(21)00758-0 - DOI - PMC - PubMed
-
- Francis PA, Fleming GF, Láng I, et al. ; SOFT Investigators and the International Breast Cancer Study Group (a division of ETOP IBCSG Partners Foundation) . Adjuvant endocrine therapy in premenopausal breast cancer: 12-Year results from SOFT. J Clin Oncol. 2023;41(7):1370-1375. doi: 10.1200/JCO.22.01065 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

