Biomarker Detection and Validation for Corneal Involvement in Patients With Acute Infectious Conjunctivitis
- PMID: 39145969
- PMCID: PMC11327903
- DOI: 10.1001/jamaophthalmol.2024.2891
Biomarker Detection and Validation for Corneal Involvement in Patients With Acute Infectious Conjunctivitis
Abstract
Importance: Infectious conjunctivitis can lead to corneal involvement and result in ocular morbidity. The identification of biomarkers associated with corneal involvement has the potential to improve patient care.
Objective: To identify biomarkers in patients with acute infectious conjunctivitis.
Design, setting, and participants: This cross-sectional study took place from December 2016 to March 2024. Analyses were performed in 3 phases. First, logistic regression and machine learning algorithms were used to predict the probability of demonstrating corneal involvement in patients with presumed infectious conjunctivitis. Second, quantitative reverse transcription polymerase chain reaction (RT-qPCR) was used to confirm the most important biomarker gene identified by the algorithm. Third, the biomarker gene was validated in prospectively collected conjunctival samples of adult patients from 3 outpatient centers in Thailand and 1 in India. Patients with signs and symptoms of infectious conjunctivitis and onset within less than 14 days were eligible. Exclusion criteria were the inability to consent, presumed toxicity, or allergic conjunctivitis.
Exposures: Acute infectious conjunctivitis.
Main outcomes and measures: The identification and validation of ocular surface gene expression associated with corneal findings on slitlamp examination.
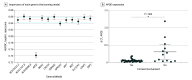
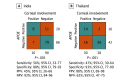
Results: Thirteen genes exhibited a 1.5-log2 fold change in expression in patients with corneal involvement compared to patients without corneal involvement. Using the 13 genes to train and cross validate, logistic regression produced the highest mean area under the receiver operating characteristic curve (AUROC; 0.85; 95% CI, 0.84-0.86) for corneal involvement. The removal of apolipoprotein E (APOE) from the gene ensemble led to a decline in predictive performance of the logistic regression classifier (from mean AUROC 0.85 [95% CI, 0.84-0.86] to 0.74 [95% CI, 0.73-0.75]; adjusted P = .001 [Tukey test]). Orthogonal testing of APOE expression level with RT-qPCR showed that APOE expression was higher in patients with corneal involvement compared to patients without (median [IQR], 0.23 [0.04-0.47] vs 0.04 [0.02-0.06]; P = .004 [Mann-Whitney U test]). Using a Youden index of 0.23 Δ threshold cycle, APOE had a sensitivity of 56% (95% CI, 33-77) and a specificity of 88% (95% CI, 79-93) in 106 samples with conjunctivitis at Aravind, India (P < .001 [Fisher exact test]). When applied to a different patient population in Thailand, the same criteria could discriminate between disease states (58 samples; sensitivity, 47%; 95% CI, 30-64 and specificity, 93%; 95% CI, 77-99; P = .001 [Fisher exact test]).
Conclusions and relevance: The results from this study suggest that the host conjunctival immune response can be meaningfully interrogated to identify biomarkers for ocular surface diseases.
Conflict of interest statement
Figures

Comment on
-
Molecular Sequencing and Biomarkers in Acute Infectious Conjunctivitis.JAMA Ophthalmol. 2024 Sep 1;142(9):872-873. doi: 10.1001/jamaophthalmol.2024.3091. JAMA Ophthalmol. 2024. PMID: 39145964 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

