Identification of pathways to high-level vancomycin resistance in Clostridioides difficile that incur high fitness costs in key pathogenicity traits
- PMID: 39146240
- PMCID: PMC11326576
- DOI: 10.1371/journal.pbio.3002741
Identification of pathways to high-level vancomycin resistance in Clostridioides difficile that incur high fitness costs in key pathogenicity traits
Abstract
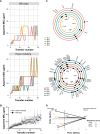
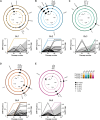
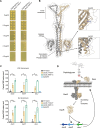
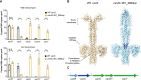
Clostridioides difficile is an important human pathogen, for which there are very limited treatment options, primarily the glycopeptide antibiotic vancomycin. In recent years, vancomycin resistance has emerged as a serious problem in several gram-positive pathogens, but high-level resistance has yet to be reported for C. difficile, although it is not known if this is due to constraints upon resistance evolution in this species. Here, we show that resistance to vancomycin can evolve rapidly under ramping selection but is accompanied by fitness costs and pleiotropic trade-offs, including sporulation defects that would be expected to severely impact transmission. We identified 2 distinct pathways to resistance, both of which are predicted to result in changes to the muropeptide terminal D-Ala-D-Ala that is the primary target of vancomycin. One of these pathways involves a previously uncharacterised D,D-carboxypeptidase, expression of which is controlled by a dedicated two-component signal transduction system. Our findings suggest that while C. difficile is capable of evolving high-level vancomycin resistance, this outcome may be limited clinically due to pleiotropic effects on key pathogenicity traits. Moreover, our data identify potential mutational routes to resistance that should be considered in genomic surveillance.
Copyright: © 2024 Buddle et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Summit Therapeutics Inc were industrial partners for JEB’s MRC DiMeN iCASE PhD studentship but had no input in study design, interpretation or manuscript preparation. The authors declare no further competing interests.
Figures


References
-
- Aguado JM, Anttila VJ, Galperine T, Goldenberg SD, Gwynn S, Jenkins D, et al.. Highlighting clinical needs in Clostridium difficile infection: the views of European healthcare professionals at the front line. J Hosp Infect. 2015;90(2):117–25. Epub 20150311. doi: 10.1016/j.jhin.2015.03.001 . - DOI - PubMed
-
- Lawley TD, Clare S, Walker AW, Goulding D, Stabler RA, Croucher N, et al.. Antibiotic treatment of Clostridium difficile carrier mice triggers a supershedder state, spore-mediated transmission, and severe disease in immunocompromised hosts. Infect Immun. 2009;77(9):3661–9. Epub 20090629. doi: 10.1128/IAI.00558-09 ; PubMed Central PMCID: PMC2737984. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

