Isolation and Crystallographic Characterization of an Octavalent Co2O2 Diamond Core
- PMID: 39146525
- PMCID: PMC12181939
- DOI: 10.1021/jacs.4c07335
Isolation and Crystallographic Characterization of an Octavalent Co2O2 Diamond Core
Abstract
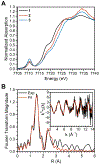
High-valent cobalt oxides play a pivotal role in alternative energy technology as catalysts for water splitting and as cathodes in lithium-ion batteries. Despite this importance, the properties governing the stability of high-valent cobalt oxides and specifically possible oxygen evolution pathways are not clear. One root of this limited understanding is the scarcity of high-valent Co(IV)-containing model complexes; there are no reports of stable, well-defined complexes with multiple Co(IV) centers. Here, an oxidatively robust fluorinated ligand scaffold enables the isolation and crystallographic characterization of a Co(IV)2-bis-μ-oxo complex. This complex is remarkably stable, in stark contrast with previously reported Co(IV)2 species that are highly reactive, which demonstrates that oxy-Co(IV)2 species are not necessarily unstable with respect to oxygen evolution. This example underscores a new design strategy for highly oxidizing transition-metal fragments and provides detailed data on a previously inaccessible chemical unit of relevance to O-O bond formation and oxygen evolution.
Figures
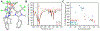

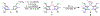
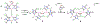
References
-
- Singh A; Spiccia L Water Oxidation Catalysts Based on Abundant 1st Row Transition Metals. Coordination Chemistry Reviews 2013, 257 (17), 2607–2622. 10.1016/j.ccr.2013.02.027. - DOI
-
- Wu Q; Zhang B; Lu Y Progress and Perspective of High-Voltage Lithium Cobalt Oxide in Lithium-Ion Batteries. Journal of Energy Chemistry 2022, 74, 283–308. 10.1016/j.jechem.2022.07.007. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

