Health-related quality of life in patients with CLDN18.2-positive, locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma: results from the SPOTLIGHT and GLOW clinical trials
- PMID: 39146670
- PMCID: PMC11374961
- DOI: 10.1016/j.esmoop.2024.103663
Health-related quality of life in patients with CLDN18.2-positive, locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma: results from the SPOTLIGHT and GLOW clinical trials
Abstract
Background: First-line zolbetuximab plus chemotherapy (SPOTLIGHT, mFOLFOX6; GLOW, CAPOX) significantly improved progression-free survival (PFS) and overall survival (OS) versus placebo plus chemotherapy in patients with human epidermal growth factor receptor 2-negative, locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma whose tumors were claudin 18 isoform 2-positive in the phase III SPOTLIGHT (NCT03504397) and GLOW (NCT03653507) studies. We present patient-reported outcomes (PROs) from these studies.
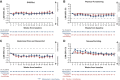
Materials and methods: Health-related quality of life (HRQoL) was measured in the full analysis sets using the European Organisation for Research and Treatment of Cancer Quality of Life of Cancer Patients Core Questionnaire (QLQ-C30) and Oesophago-Gastric Module (QLQ-OG25), Global Pain, and 5-level EQ-5D (EQ-5D-5L) questionnaires. Analyses focused on key PRO domains: global health status (GHS)/QoL, physical functioning, abdominal pain and discomfort, and nausea/vomiting. Least squares mean (LSM) changes from baseline and time to first definitive deterioration (TTDD) were evaluated combined across SPOTLIGHT and GLOW and for individual studies. Time to confirmed deterioration (TTCD) was evaluated independently for SPOTLIGHT and GLOW.
Results: The combined analysis set included 1072 patients (zolbetuximab plus chemotherapy, 537; placebo plus chemotherapy, 535). Compliance rates were similar between treatment arms. Similar trends were observed in the zolbetuximab versus placebo arms for LSM changes from baseline in key PRO domains, with no clinically meaningful deterioration. Nausea/vomiting worsened during the first few zolbetuximab cycles but later returned to baseline levels. Overall TTCD and TTDD results were similar between arms in both studies.
Conclusions: Patients in SPOTLIGHT and GLOW maintained measured HRQoL relative to baseline when treated with first-line zolbetuximab added to chemotherapy. Zolbetuximab plus chemotherapy improved PFS and OS without negatively affecting HRQoL in key PRO domains compared with placebo plus chemotherapy.
Keywords: CLDN18.2; gastric adenocarcinoma; gastroesophageal junction adenocarcinoma; patient-reported outcomes; quality of life; zolbetuximab.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Smyth E.C., Nilsson M., Grabsch H.I., van Grieken N.C., Lordick F. Gastric cancer. Lancet. 2020;396(10251):635–648. - PubMed
-
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
-
- Shah M.A., Kennedy E.B., Alarcon-Rozas A.E., et al. Immunotherapy and targeted therapy for advanced gastroesophageal cancer: ASCO guideline. J Clin Oncol. 2023;41(7):1470–1491. - PubMed
-
- Lordick F., Carneiro F., Cascinu S., et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):1005–1020. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

