The calcium-sensing-receptor (CaSR) in adipocytes contributes to sex-differences in the susceptibility to high fat diet induced obesity and atherosclerosis
- PMID: 39146692
- PMCID: PMC11379552
- DOI: 10.1016/j.ebiom.2024.105293
The calcium-sensing-receptor (CaSR) in adipocytes contributes to sex-differences in the susceptibility to high fat diet induced obesity and atherosclerosis
Abstract
Background: Female mice are more resistant to obesogenic effects of a high-fat diet (HFD), compared to male mice. Although the underlying mechanisms are poorly understood, sex hormones seem to play an important role. Interestingly, the activity of the oestrogen receptor-α (ERα) is affected by the calcium-sensing-receptor (CaSR). Therefore, we investigated sex-differences upon diet-induced obesity and the role of adipocyte-specific CaSR herein.
Methods: Adipocyte-specific Casr deficient mice (AdipoqCre+Casrflox) and control mice (Casrflox) were injected with AAV8-PCSK9 to make them prone to develop atherosclerosis and fed an obesity-inducing diet for 12 weeks.
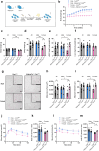
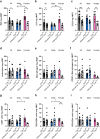
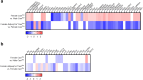
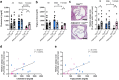
Findings: Female mice have lower visceral white adipose tissue (vWAT) mass compared to male mice, while this sex-difference is abolished upon adipocyte-specific Casr deficiency. Furthermore, while females showed elevated levels of inflammatory cytokines and CD3+CD8+ T cell accumulation in vWAT, compared to males, adipocyte-specific Casr deficiency abrogated this sex-phenotype and demonstrated an inhibition of inflammatory signalling pathways. The expression of Erα, as well as associated genes involved in adipocyte differentiation, was increased in female mice in a mostly adipocyte-specific Casr dependent manner. Interestingly, circulating lipid levels were reduced in female compared to male mice, which correlated with decreased atherosclerotic plaque formation. These systemic effects were abrogated upon adipocyte-specific Casr deficiency.
Interpretation: Our findings indicate that female mice show a more pronounced vWAT dysfunction compared to males upon obesity. This sex effect is abolished upon adipocyte-specific Casr deficiency. In contrast, females show diminished atherosclerotic plaque formation compared to males, an effect that was abrogated by adipocyte-specific Casr deficiency.
Funding: This work was supported by a grant from the Interdisciplinary Center for Clinical Research within the faculty of Medicine at the RWTH Aachen University, by the Corona Foundation, by the Deutsche Forschungsgemeinschaft (DFG), the BMBF and Free State of Bavaria and the DZHK.
Keywords: Adipocytes; Atherosclerosis; Calcium-sensing receptor; Inflammation; Obesity; Sex-differences.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests None.
Figures

References
-
- Knopp R.H., Paramsothy P., Retzlaff B.M., et al. Gender differences in lipoprotein metabolism and dietary response: basis in hormonal differences and implications for cardiovascular disease. Curr Atheroscler Rep. 2005;7(6):472–479. - PubMed
-
- Menegoni F., Galli M., Tacchini E., Vismara L., Cavigioli M., Capodaglio P. Gender-specific effect of obesity on balance. Obesity. 2009;17(10):1951–1956. - PubMed
-
- Gerdts E., Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nat Med. 2019;25(11):1657–1666. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

