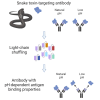
Engineering of pH-dependent antigen binding properties for toxin-targeting IgG1 antibodies using light-chain shuffling
- PMID: 39146931
- PMCID: PMC11385703
- DOI: 10.1016/j.str.2024.07.014
Engineering of pH-dependent antigen binding properties for toxin-targeting IgG1 antibodies using light-chain shuffling
Abstract
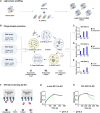
Immunoglobulin G (IgG) antibodies that bind their cognate antigen in a pH-dependent manner (acid-switched antibodies) can release their bound antigen for degradation in the acidic environment of endosomes, while the IgGs are rescued by the neonatal Fc receptor (FcRn). Thus, such IgGs can neutralize multiple antigens over time and therefore be used at lower doses than their non-pH-responsive counterparts. Here, we show that light-chain shuffling combined with phage display technology can be used to discover IgG1 antibodies with increased pH-dependent antigen binding properties, using the snake venom toxins, myotoxin II and α-cobratoxin, as examples. We reveal differences in how the selected IgG1s engage their antigens and human FcRn and show how these differences translate into distinct cellular handling properties related to their pH-dependent antigen binding phenotypes and Fc-engineering for improved FcRn binding. Our study showcases the complexity of engineering pH-dependent antigen binding IgG1s and demonstrates the effects on cellular antibody-antigen recycling.
Keywords: Antibody recycling; FcRn; HERA; acid-switched antibodies; human endothelial cell-based recycling assay; light-chain shuffling; myotoxin II; pH-dependent antigen binding properties; phage display technology; snake venom; α-cobratoxin.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

