Trained immunity suppression determines kidney allograft survival
- PMID: 39147201
- PMCID: PMC11789421
- DOI: 10.1016/j.ajt.2024.08.006
Trained immunity suppression determines kidney allograft survival
Abstract
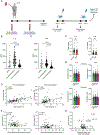
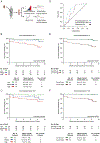
The innate immune system plays an essential role in regulating the immune responses to kidney transplantation, but the mechanisms through which innate immune cells influence long-term graft survival are unclear. The current study highlights the vital role of trained immunity in kidney allograft survival. Trained immunity describes the epigenetic and metabolic changes that innate immune cells undergo following an initial stimulus, allowing them have a stronger inflammatory response to subsequent stimuli. We stimulated healthy peripheral blood mononuclear cells with pretransplant and posttransplant serum of kidney transplant patients and immunosuppressive drugs in an in vitro trained immunity assay and measured tumor necrosis factor and interleukin 6 cytokine levels in the supernatant as a readout for trained immunity. We show that the serum of kidney transplant recipients collected 1 week after transplantation can suppress trained immunity. Importantly, we found that kidney transplant recipients whose serum most strongly suppressed trained immunity rarely experienced graft loss. This suppressive effect of posttransplant serum is likely mediated by previously unreported effects of immunosuppressive drugs. Our findings provide mechanistic insights into the role of innate immunity in kidney allograft survival, uncovering trained immunity as a potential therapeutic target for improving graft survival.
Keywords: graft survival; innate immunity; kidney transplantation; trained immunity.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. L. A. B. Joosten is scientific founder of TTxD, LembaTX, and SalvinaTX. M. G. Netea is scientific founder of TTxD and Biotrip. W. J. M. Mulder is scientific founder of TTxD and Biotrip. Other authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

