C5aR1 antagonism suppresses inflammatory glial responses and alters cellular signaling in an Alzheimer's disease mouse model
- PMID: 39147742
- PMCID: PMC11327341
- DOI: 10.1038/s41467-024-51163-6
C5aR1 antagonism suppresses inflammatory glial responses and alters cellular signaling in an Alzheimer's disease mouse model
Abstract
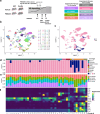
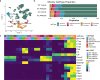
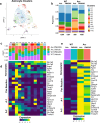
Alzheimer's disease (AD) is the leading cause of dementia in older adults, and the need for effective, sustainable therapeutic targets is imperative. The complement pathway has been proposed as a therapeutic target. C5aR1 inhibition reduces plaque load, gliosis, and memory deficits in animal models, however, the cellular bases underlying this neuroprotection were unclear. Here, we show that the C5aR1 antagonist PMX205 improves outcomes in the Arctic48 mouse model of AD. A combination of single cell and single nucleus RNA-seq analysis of hippocampi derived from males and females identified neurotoxic disease-associated microglia clusters in Arctic mice that are C5aR1-dependent, while microglial genes associated with synapse organization and transmission and learning were overrepresented in PMX205-treated mice. PMX205 also reduced neurotoxic astrocyte gene expression, but clusters associated with protective responses to injury were unchanged. C5aR1 inhibition promoted mRNA-predicted signaling pathways between brain cell types associated with cell growth and repair, while suppressing inflammatory pathways. Finally, although hippocampal plaque load was unaffected, PMX205 prevented deficits in short-term memory in female Arctic mice. In conclusion, C5aR1 inhibition prevents cognitive loss, limits detrimental glial polarization while permitting neuroprotective responses, as well as leaving most protective functions of complement intact, making C5aR1 antagonism an attractive therapeutic strategy for AD.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Update of
-
C5aR1 antagonism suppresses inflammatory glial gene expression and alters cellular signaling in an aggressive Alzheimer's model.bioRxiv [Preprint]. 2023 Aug 22:2023.08.22.554306. doi: 10.1101/2023.08.22.554306. bioRxiv. 2023. Update in: Nat Commun. 2024 Aug 15;15(1):7028. doi: 10.1038/s41467-024-51163-6. PMID: 37662399 Free PMC article. Updated. Preprint.
References
-
- Association As. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement.18, 700–789 (2022). - PubMed
MeSH terms
Substances
Grants and funding
- 2021-A-020-FEL/Larry L. Hillblom Foundation (Larry L. Hillblom Foundation, Inc.)
- T32 AG00096/U.S. Department of Health & Human Services | NIH | Office of Extramural Research, National Institutes of Health (OER)
- AARFD-20-677771/ALZ/Alzheimer's Association/United States
- T32 AG000096/AG/NIA NIH HHS/United States
- P30 CA062203/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

