A broadly protective antibody targeting glycoprotein Gn inhibits severe fever with thrombocytopenia syndrome virus infection
- PMID: 39147753
- PMCID: PMC11327358
- DOI: 10.1038/s41467-024-51108-z
A broadly protective antibody targeting glycoprotein Gn inhibits severe fever with thrombocytopenia syndrome virus infection
Abstract
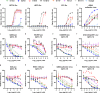
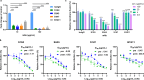
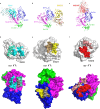
Severe fever with thrombocytopenia syndrome virus (SFTSV) is an emerging bunyavirus that causes severe viral hemorrhagic fever and thrombocytopenia syndrome with a fatality rate of up to 30%. No licensed vaccines or therapeutics are currently available for humans. Here, we develop seven monoclonal antibodies (mAbs) against SFTSV surface glycoprotein Gn. Mechanistic studies show that three neutralizing mAbs (S2A5, S1G3, and S1H7) block multiple steps during SFTSV infection, including viral attachment and membrane fusion, whereas another neutralizing mAb (B1G11) primarily inhibits the viral attachment step. Epitope binning and X-ray crystallographic analyses reveal four distinct antigenic sites on Gn, three of which have not previously been reported, corresponding to domain I, domain II, and spanning domain I and domain II. One of the most potent neutralizing mAbs, S2A5, binds to a conserved epitope on Gn domain I and broadly neutralizes infection of six SFTSV strains corresponding to genotypes A to F. A single dose treatment of S2A5 affords both pre- and post-exposure protection of mice against lethal SFTSV challenge without apparent weight loss. Our results support the importance of glycoprotein Gn for eliciting a robust humoral response and pave a path for developing prophylactic and therapeutic antibodies against SFTSV infection.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

