A potential DES catalyst for the fast and green synthesis of benzochromenopyrimidines and pyranopyrimidines
- PMID: 39147849
- PMCID: PMC11327281
- DOI: 10.1038/s41598-024-69817-2
A potential DES catalyst for the fast and green synthesis of benzochromenopyrimidines and pyranopyrimidines
Abstract
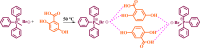
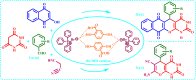
A gentisic acid based-Deep Eutectic Solvent (MTPPBr/GA-DES) was synthesized by mixing one mole of methyl triphenylphosphonium bromide (MTPPBr) and one mole of gentisic acid (GA: 2,5-dihydroxy-benzoic acid) based on the eutectic point phase diagram. Then, it was characterized by FT-IR, NMR, densitometer, and TGA/DTA techniques and used as a potent and novel catalyst for the fast and green synthesis of: (i) Five new 2(a-e) and five known 2(f-j) benzo[6,7]chromeno[2,3-d]pyrimidines and (ii) One new (3a) and eleven known 3(b-l) pyrano[2,3-d]pyrimidines, in solvent-free conditions, short reaction times, and high yields. It is important to mention that for the synthesis of 2(a-j), there is only one reference which states that the reaction times are extremely long (720-2400 min), while these times are reduced to approximately 35-50 min in our proposed strategy, indicatinging that the rate of reactions will be 20-48 times faster, which is the clear and most obvious advantage of our approach.
Keywords: Deep eutectic solvent; Gentisic acid; Green synthesis; benzo[6,7]chromeno[2,3-d]pyrimidines; pyrano[2,3-d]pyrimidines.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Abbott, A. P. et al. Preparation of novel, moisture-stable, Lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains electronic supplementary information (ESI) available: Plot of conductivity vs. temperature for the ionic liquid. Chem. Commun.1, 2010–2011 (2001).10.1039/b106357j - DOI - PubMed
-
- Walden, P. Molecular weights and electrical conductivity of several fused salts. Bulletin Acad. Imper. Sci.1800, 405–422 (1914).
-
- Paiva, A. et al. Natural deep eutectic solvents–solvents for the 21st century. ACS Sustain. Chem. Eng.2, 1063–1071 (2014).10.1021/sc500096j - DOI
LinkOut - more resources
Full Text Sources

