Unveiling the Burden of Drug-Induced Impulsivity: A Network Analysis of the FDA Adverse Event Reporting System
- PMID: 39147961
- PMCID: PMC11554833
- DOI: 10.1007/s40264-024-01471-z
Unveiling the Burden of Drug-Induced Impulsivity: A Network Analysis of the FDA Adverse Event Reporting System
Abstract
Introduction: Impulsivity induced by dopaminergic agents, like pramipexole and aripiprazole, can lead to behavioral addictions that impact on social functioning and quality of life of patients and families (e.g., resulting in unemployment, marital problems, anxiety). These secondary effects, interconnected in networks of signs and symptoms, are usually overlooked by clinical trials, not reported in package inserts, and neglected in clinical practice.
Objective: This study explores the syndromic burden of impulsivity induced by pramipexole and aripiprazole, pinpointing key symptoms for targeted mitigation.
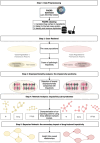
Methods: An event-event Information Component (IC) on the FDA Adverse Event Reporting System (FAERS) (January 2004 to March 2022) identified the syndrome of events disproportionally co-reported with impulsivity, separately for pramipexole and aripiprazole. A greedy-modularity clustering on composite network analyses (positive pointwise mutual information [PPMI], Ising, Φ) identified sub-syndromes. Bayesian network modeling highlighted possible precipitating events.
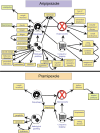
Results: Suspected drug-induced impulsivity was documented in 7.49% pramipexole and 4.50% aripiprazole recipients. The highest IC concerned obsessive-compulsive disorder (reporting rate = 26.77%; IC median = 3.47, 95% confidence interval [CI] = 3.33-3.57) and emotional distress (21.35%; 3.42, 3.26-3.54) for pramipexole, bankruptcy (10.58%; 4.43, 4.26-4.55) and divorce (7.59%; 4.38, 4.19-4.53) for aripiprazole. The network analysis identified delusional jealousy and dopamine dysregulation sub-syndromes for pramipexole, obesity-hypoventilation and social issues for aripiprazole. The Bayesian network highlighted anxiety and economic problems as potentially precipitating events.
Conclusion: The under-explored consequences of drug-induced impulsivity significantly burden patients and families. Network analyses, exploring syndromic reactions and potential precipitating events, complement traditional techniques and clinical judgment. Characterizing the secondary impact of reactions will support informed patient-centered decision making.
© 2024. The Author(s).
Conflict of interest statement
Figures




References
-
- Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129–36. - PubMed
-
- Young A. The anthropologies of illness and sickness. Annu Rev Anthropol. 1982;11:257–85.
-
- Kleinman A, Eisenberg L, Good B. Culture, illness, and care: clinical lessons from anthropologic and cross-cultural research. Ann Intern Med. 1978;88:251–8. - PubMed
-
- Lorimer S, Cox A, Langford NJ. A patient’s perspective: the impact of adverse drug reactions on patients and their views on reporting. J Clin Pharm Ther. 2012;37:148–52. - PubMed
-
- Suh D-C, Woodall BS, Shin S-K, Santis ERH-D. Clinical and economic impact of adverse drug reactions in hospitalized patients. Ann Pharmacother. 2000;34:1373–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

