Converting PROMIS®-29 v2.0 profile data to SF-36 physical and mental component summary scores in patients with cardiovascular disorders
- PMID: 39148105
- PMCID: PMC11328444
- DOI: 10.1186/s12955-024-02277-4
Converting PROMIS®-29 v2.0 profile data to SF-36 physical and mental component summary scores in patients with cardiovascular disorders
Abstract
Background: Health-related quality of life (HRQL) has become an important outcome parameter in cardiology. The MOS 36-ltem Short-Form Health Survey (SF-36) and the PROMIS-29 are two widely used generic measures providing composite HRQL scores. The domains of the SF-36, a well-established instrument utilized for several decades, can be aggregated to physical (PCS) and mental (MCS) component summary scores. Alternative scoring algorithms for correlated component scores (PCSc and MCSc) have also been suggested. The PROMIS-29 is a newer but increasingly used HRQL measure. Analogous to the SF-36, physical and mental health summary scores can be derived from PROMIS-29 domain scores, based on a correlated factor solution. So far, scores from the PROMIS-29 are not directly comparable to SF-36 results, complicating the aggregation of research findings. Thus, our aim was to provide algorithms to convert PROMIS-29 data to well-established SF-36 component summary scores.
Methods: Data from n = 662 participants of the Berlin Long-term Observation of Vascular Events (BeLOVE) study were used to estimate linear regression models with either PROMIS-29 domain scores or aggregated PROMIS-29 physical/mental health summary scores as predictors and SF-36 physical/mental component summary scores as outcomes. Data from a subsequent assessment point (n = 259) were used to evaluate the agreement between empirical and predicted SF-36 scores.
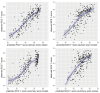
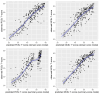
Results: PROMIS-29 domain scores as well as PROMIS-29 health summary scores showed high predictive value for PCS, PCSc, and MCSc (R2 ≥ 70%), and moderate predictive value for MCS (R2 = 57% and R2 = 40%, respectively). After applying the regression coefficients to new data, empirical and predicted SF-36 component summary scores were highly correlated (r > 0.8) for most models. Mean differences between empirical and predicted scores were negligible (|SMD|<0.1).
Conclusions: This study provides easy-to-apply algorithms to convert PROMIS-29 data to well-established SF-36 physical and mental component summary scores in a cardiovascular population. Applied to new data, the agreement between empirical and predicted SF-36 scores was high. However, for SF-36 mental component summary scores, considerably better predictions were found under the correlated (MCSc) than under the original factor model (MCS). Additionally, as a pertinent byproduct, our study confirmed construct validity of the relatively new PROMIS-29 health summary scores in cardiology patients.
Keywords: Cardiovascular diseases; Health composite scores; Health-related quality of life; Mapping; Outcome measures; PROMIS-29; Patient-reported outcomes; SF-36.
© 2024. The Author(s).
Conflict of interest statement
Frank Edelmann (FE) reports grants from German Research Foundation (DFG), grants from German Ministry of Education and Research, grants from the German Herta Foundation; during the conduct of the study; personal fees and non-financial support from Novartis, grants and personal fees from Boehringer Ingelheim, personal fees from CVRx, Pfizer, Medtronic, grants and personal fees from Servier, personal fees from MSD, personal fees from Merck & Co., grants from AstraZeneca, personal fees from Bayer, personal fees from Resmed, personal fees from Berlin Chemie, grants from Thermo Fischer, personal fees from Vifor Pharma, personal fees from PharmaCosmos outside the submitted work.Matthias Endres (ME) reports grants from Bayer and fees paid to the Charité from Abbot, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo, Amgen, Sanofi, , Novartis, Pfizer, all outside the submitted work. ME received funding from DFG under Germany´s Excellence Strategy – EXC-2049 – 390688087, Collaborative Research Center ReTune TRR 295- 424778381, BMBF, DZNE, DZHK, EU, Corona Foundation, and Fondation Leducq.Holger Gerhardt (HG) reports grants from the DFG, the Leducq Foundation, the BMBF and the German Center for Cardiovascular Research (DZHK) during the conduct of the study, outside of the submitted work.Ulf Landmesser (UL) reports research funding from German Cardiovascular Research Network (DZHK); Fondation Leducq; research grants from Novartis, Bayer and AmgenKnut Mai (KM) declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.Gregor Liegl (GL), received funding for research from the German Research Foundation (DFG).Dominik N. Müller (DNM) received funding for research from Bayer Healthcare, Deutsche Forschungsgemeinschaft and from BMBF.Christian H Nolte (CHN) received research grants from German Ministry of Research and Education, German Center for neurodegenerative Diseases (DZNE), German Center for cardiovascular Research (DZHK), and speaker and/or consultation fees from Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer Pharma, Abbott, Novartis, Daichii-Sankyo and Alexion all outside the submitted work.Tobias Pischon (TP) received grants from the Federal Ministry of Education and Research (BMBF), the Federal Ministry of Food and Agriculture (BMEL), the Federal Ministry for Economic Affairs and Energy (BMWi), the Deutsche Forschungsgemeinschaft (DFG), Deutsche Herzstiftung, German Academic Exchange Service (DAAD).Kai M. Schmidt-Ott (KMSO) reports receiving research funding from Deutsche Forschungsgemeinschaft (CRC 1365, GRK 2318, RU 2841), Urological Research Foundation Berlin, ERA PerMed (OnAKI-ICI), COVID-19-Forschungsnetzwerk Niedersachsen (EXTINCT post COVID), Niedersächsisches Ministerium für Wissenschaft und Kultur (zukunft.Niedersachsen), FAST BioMedical, Quark Pharmaceuticals, REATA, Boehringer Ingelheim, AstraZeneca, Sanofi, Alentis, Roche, and Alexion; having received consultancy fees from BioPorto Diagnostics and Abionyx; having participated in advisory boards with Bayer, Stadapharm, and Boehringer Ingelheim; having received license revenue related to the use of a neutrophil gelatinase- associated lipocalin assay via Columbia University; and being an editorial board member of Kidney International and Kidney International Reports; each outside the submitted work.Jeanette Schulz-Menger (JSM) reports grants from Bayer Healthcare, non-financial support from Siemens healthineers, non-financial support from Circle cardiovascular, non-financial support from Medis, outside the submitted work, and Bayer Healthcare, Advisor. Furthermore, funding for research from the EU, DZHK, Deutsche Herzstiftung.Jochen Spranger (JS) received funding for research from Deutsche Forschungsgemeinschaft and from BMBF.Martin Witzenrath (MW) received funding for research from Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung, Deutsche Gesellschaft für Pneumologie, European Respiratory Society, Marie Curie Foundation, Else Kröner Fresenius Foundation, Capnetz Foundation, International Max Planck Research School, Actelion, Bayer Health Care, Biotest, Boehringer Ingelheim, Noxxon, Pantherna, Quark Pharma, Vaxxilon, and for lectures and advisory from Actelion, Aptarion, Astra Zeneca, Bayer Health Care, Berlin Chemie, Biotest, Boehringer Ingelheim, Chiesi, Glaxo Smith Kline, Novartis, Noxxon, Pantherna, Teva und Vaxxilon.All remaining authors do not report potential conflicts of interest.
Figures
Similar articles
-
Correlated physical and mental health summary scores for the SF-36 and SF-12 Health Survey, V.I.Health Qual Life Outcomes. 2007 Sep 7;5:54. doi: 10.1186/1477-7525-5-54. Health Qual Life Outcomes. 2007. PMID: 17825096 Free PMC article.
-
PROMIS measures can be used to assess symptoms and function in long-term hematopoietic cell transplantation survivors.Cancer. 2018 Feb 15;124(4):841-849. doi: 10.1002/cncr.31089. Epub 2017 Oct 26. Cancer. 2018. PMID: 29072787 Free PMC article.
-
The Relationship Among 3 Generic Patient-Reported Outcome Instruments in Patients With Lower Extremity Health Conditions.J Athl Train. 2019 May;54(5):550-555. doi: 10.4085/1062-6050-350-17. Epub 2019 May 14. J Athl Train. 2019. PMID: 31084504 Free PMC article.
-
The sensitivity of the MOS SF-12 and PROMIS® global summary scores to adverse health events in an older cohort.Qual Life Res. 2018 Aug;27(8):2207-2215. doi: 10.1007/s11136-018-1871-y. Epub 2018 May 3. Qual Life Res. 2018. PMID: 29725968
-
Scoring the SF-36 in Orthopaedics: A Brief Guide.J Bone Joint Surg Am. 2015 Oct 7;97(19):1628-34. doi: 10.2106/JBJS.O.00030. J Bone Joint Surg Am. 2015. PMID: 26446970 Free PMC article. Review.
Cited by
-
Impact of Post-Procedural Atrial Arrhythmia on Long-Term Cardiac Function and Quality of Life Following Patent Foramen Ovale Closure.Cardiol Res. 2025 Jul 28;16(4):373-379. doi: 10.14740/cr2105. eCollection 2025 Aug. Cardiol Res. 2025. PMID: 40809734 Free PMC article.
-
Towards Standardized Assessment of Outcomes in Back Pain-Validation of Linking Studies Between Disease-Specific and Generic Patient-Reported Outcome Measures.J Clin Med. 2024 Oct 30;13(21):6524. doi: 10.3390/jcm13216524. J Clin Med. 2024. PMID: 39518663 Free PMC article.
References
-
- Burns DJP, Arora J, Okunade O, Beltrame JF, Bernardez-Pereira S, Crespo-Leiro MG, et al. International Consortium for Health Outcomes Measurement (ICHOM): standardized patient-centered outcomes measurement set for heart failure patients. JACC Heart Fail. 2020;8(3):212–22. 10.1016/j.jchf.2019.09.007 - DOI - PMC - PubMed
-
- Hahn EA, Walsh MN, Allen LA, Lee CS, Denfeld QE, Teuteberg JJ, et al. Validity of patient-reported outcomes Measurement Information System Physical, Mental, and Social Health measures after Left Ventricular assist device implantation and implications for patient care. Circ Cardiovasc Qual Outcomes. 2023;16(2):e008690. 10.1161/CIRCOUTCOMES.121.008690 - DOI - PMC - PubMed
-
- Marquis P, Caron M, Emery M-P, Scott JA, Arnould B, Acquadro C. The role of Health-Related Quality of Life Data in the drug approval processes in the US and Europe. Pharm Med. 2011;25(3):147–60.10.1007/BF03256856 - DOI
-
- Kornowski R. Patient-reported outcome measures in cardiovascular disease. Eur Heart J - Qual Care Clin Outcomes. 2021. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

