Regulatory Pathways for Qualification and Acceptance of Digital Health Technology-Derived Clinical Trial Endpoints: Considerations for Sponsors
- PMID: 39148198
- PMCID: PMC11652808
- DOI: 10.1002/cpt.3398
Regulatory Pathways for Qualification and Acceptance of Digital Health Technology-Derived Clinical Trial Endpoints: Considerations for Sponsors
Abstract
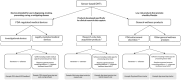
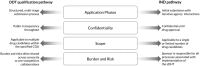
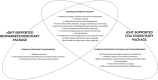
Despite widespread interest and substantial investment in the adoption of sensor-based digital health technologies (sDHTs) for remote data capture in drug development trials, no drug has been approved based on an sDHT-derived primary endpoint in the United States (US). One reason for this lack of advancement is the complexity of obtaining regulatory endorsement for those endpoints within current US regulatory pathways. The goal of our review is to describe the two choices currently available to pharmaceutical study Sponsors: (i) they may navigate the traditional route of compiling the evidence to support the sDHT-derived endpoint in their investigational new drug (IND) application, requiring specific expertise and substantial resources; or (ii) they may navigate the drug development tool (DDT) pathway with the goal of qualifying their sDHT-derived endpoint as a biomarker or clinical outcome assessment applicable to a broader context of use (COU), either alone or as part of a partnership or consortium. We describe the nuances of each pathway; the evidentiary requirements for supporting an sDHT-derived endpoint and the technology used to capture it; and the impact that an sDHT's regulatory status may have on a Sponsor's decision to use it for data capture. By systematically comparing the IND and DDT pathways, our over-arching goals are to support the increasing deployment of sDHTs within the clinical research setting and help advance regulatory science in the field of digital medicine.
© 2024 The Author(s). Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
JPB reports financial interests in Apnimed, Philips, Signifier Medical Technologies, and Koneksa Health, along with employment at non‐profit organizations over the period this review was written (Digital Medicine Society; Brigham and Women's Hospital; Harvard Medical School). ESI and JAW are employees of Koneksa Health and may own company stock. AC is an employee of F. Hoffmann‐La Roche Ltd and may own company stock. All other authors declared no competing interests for this work.
Figures
References
-
- Digital Medicine Society . Library of digital endpoints <https://dimesociety.org/library‐of‐digital‐endpoints/>. Accessed February 14, 2024.
-
- Food and Drug Administration . NDA approval for Aleve PM (naproxen sodium 220 mg and diphenhydramine hydrochloride 25 mg) tablets <https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2014/205352Orig...>. Accessed February 14, 2024.
-
- Food and Drug Administration . DNP clinical review <https://www.fda.gov/media/88284/download>. Accessed February 14, 2024.
-
- Food and Drug Administration . 21st Century Cures Act Deliverables <https://www.fda.gov/regulatory‐information/21st‐century‐cures‐act/21st‐c...>. Accessed February 14, 2024.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

