Blood-based biomarkers of neuronal and glial injury in active major neuropsychiatric systemic lupus erythematosus
- PMID: 39148457
- PMCID: PMC11405133
- DOI: 10.1177/09612033241272961
Blood-based biomarkers of neuronal and glial injury in active major neuropsychiatric systemic lupus erythematosus
Abstract
Background: Neuropsychiatric systemic lupus erythematosus (NPSLE) is a poorly understood and heterogeneous manifestation of SLE. Common major NPSLE syndromes include strokes, seizures, myelitis, and aseptic meningitis. Easily obtainable biomarkers are needed to assist in early diagnosis and improve outcomes for NPSLE. A frequent end-result of major syndromes is neuronal or glial injury. Blood-based neurofilament light (NfL) and glial fibrillary acidic protein (GFAP) have been utilized as markers for monitoring disease activity and/or severity in other neurodegenerative and neuroinflammatory diseases; however, they have not been evaluated in active major NPSLE.
Methods: This was a case-control study. We enrolled patients aged 12-60 years with active major NPSLE, SLE without active major NPSLE, and healthy controls. Active NPSLE was defined as being <6 months from last new or worsening neuropsychiatric symptom. Demographics, clinical data, and serum or plasma biosamples were collected.
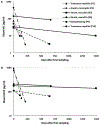
Results: Thirteen patients with active major NPSLE, 13 age/sex/kidney function matched SLE controls without active major NPSLE, and 13 age/sex matched healthy controls (mean ages 26.8, 27.3, 26.6 years) were included. 92% of each group were female. Major syndromes included stroke (5), autonomic disorder (3), demyelinating disease (2), aseptic meningitis (2), sensorimotor polyneuropathy (2), cranial neuropathy (1), seizures (1), and myelopathy (2). Mean (standard deviation) blood NfL and GFAP were 3.6 pg/ml (2.0) and 50.4 pg/ml (15.0), respectively, for the healthy controls. Compared to healthy controls, SLE without active major NPSLE had mean blood NfL and GFAP levels 1.3 pg/ml (p = .42) and 1.2 pg/ml higher (p = .53), respectively. Blood NfL was on average 17.9 pg/ml higher (95% CI: 9.2, 34.5; p < .001) and blood GFAP was on average 3.2 pg/ml higher (95% CI: 1.9, 5.5; p < .001) for cases of active major NPSLE compared to SLE without active major NPSLE. In a subset of 6 patients sampled at multiple time points, blood NfL and GFAP decreased after immunotherapy.
Conclusions: Blood NfL and GFAP levels are elevated in persons with SLE with active major NPSLE compared to disease matched controls and may lower after immunotherapy initiation. Larger and longitudinal studies are needed to ascertain their utility in a clinical setting.
Keywords: Neuropsychiatric lupus; acidic protein; biomarkers; glial fibrillary; neurofilament light.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
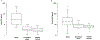
References
-
- Govoni M, Bombardieri S, Bortoluzzi A, et al. Factors and comorbidities associated with first neuropsychiatric event in systemic lupus erythematosus: does a risk profile exist? A large multicentre retrospective cross-sectional study on 959 Italian patients. Rheumatology (Oxford) 2012; 51: 157–168. 20111110. - PubMed
-
- Zhu TY, Tam LS, Lee VW, et al. Systemic lupus erythematosus with neuropsychiatric manifestation incurs high disease costs: a cost-of-illness study in Hong Kong. Rheumatology (Oxford) 2009; 48: 564–568. 20090305. - PubMed
-
- Fanouriakis A, Boumpas DT and Bertsias GK. Pathogenesis and treatment of CNS lupus. Curr Opin Rheumatol 2013; 25: 577–583. - PubMed
-
- Zirkzee EJ, Huizinga TW, Bollen EL, et al. Mortality in neuropsychiatric systemic lupus erythematosus (NPSLE). Lupus 2014; 23: 31–38. 20131115. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

