Recent applications of coinage metal nanoparticles passivated with salicylaldehyde and salicylaldehyde-based Schiff bases
- PMID: 39148500
- PMCID: PMC11322903
- DOI: 10.1039/d4na00427b
Recent applications of coinage metal nanoparticles passivated with salicylaldehyde and salicylaldehyde-based Schiff bases
Abstract
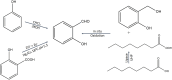
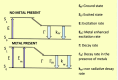
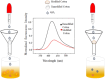
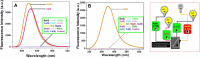
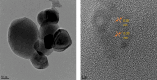
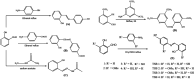
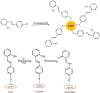
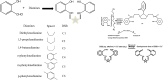
Salicylaldehyde (SD) and its derivatives are effective precursors for generating coinage metal (gold, silver, and copper) nanoparticles (NPs). These NPs have a variety of potential environmental applications, such as in water purification and sensing, and those arising from their antibacterial activity. The use of SD and its derivatives for synthesizing coinage NPs is attractive due to several factors. First, SD is a relatively inexpensive and readily available starting material. Second, the synthetic procedures are typically simple and can be carried out under mild conditions. Finally, the resulting NPs can be tailored to have specific properties, such as size, shape, and surface functionality, by varying the reaction conditions. In an alkaline solution, the phenolate form of SD was converted to its quinone form, while ionic coinage metal salts were converted to zero-valent nanoparticles. The capping in situ produced quinone of coinage metal nanoparticles generated metal-enhanced fluorescence under suitable experimental conditions. The formation of iminic bonds during the formation of Schiff bases altered the properties (especially metal-enhanced fluorescence) and applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures









References
-
- Maher K. A. Mohammes S. R. Int. J. ChemTech Res. 2015;8:937–943.
-
- Das M. Shim K. H. An S. S. A. Yi D. K. Toxicol. Environ. Health Sci. 2011;3:193–205. doi: 10.1007/s13530-011-0109-y. - DOI
-
- Khandel P. Shahi S. K. J. Nanostruct. Chem. 2018;8:369–391. doi: 10.1007/s40097-018-0285-2. - DOI
-
- Campisi S. Schiavoni M. Chan-Thaw C. E. Villa A. Catalysts. 2016;6:1–21. doi: 10.3390/catal6120185. - DOI
-
- Niu Z. Li Y. Chem. Mater. 2014;26:72–83. doi: 10.1021/cm4022479. - DOI
Publication types
LinkOut - more resources
Full Text Sources

