Bergenin mitigates neuroinflammatory damage induced by high glucose: insights from Zebrafish, murine microbial cell line, and rat models
- PMID: 39148536
- PMCID: PMC11324488
- DOI: 10.3389/fphar.2024.1339178
Bergenin mitigates neuroinflammatory damage induced by high glucose: insights from Zebrafish, murine microbial cell line, and rat models
Abstract
Background: The escalating global burden of diabetes and its associated cognitive impairment underscores the urgency for effective interventions. Bergenin shows promise in regulating glucose metabolism, mitigating inflammation, and improving cognitive function. Zebrafish models offer a unique platform for assessing drug efficacy and exploring pharmacological mechanisms, complemented by subsequent investigations in cell and rat models.
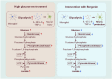
Methods: The experimental subjects included zebrafish larvae (CZ98:Tg (mpeg1:EGFP) ihb20Tg/+ ), adult zebrafish (immersed in 2% glucose), BV2 cell line (50 mM glucose + 10 μm Aβ1-42), and a streptozotocin (STZ) bilateral intracerebroventricular injection rat model. Bergenin's effects on the toxicity, behavior, and cognitive function of zebrafish larvae and adults were evaluated. The Morris water maze assessed cognitive function in rats. Neuronal histopathological changes were evaluated using HE and Nissl staining. qPCR and Western blot detected the expression of glycolysis enzymes, inflammatory factors, and Bergenin's regulation of PPAR/NF-κB pathway in these three models.
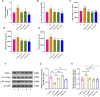
Results: 1) In zebrafish larvae, Bergenin interventions significantly reduced glucose levels and increased survival rates while decreasing teratogenicity rates. Microglial cell fluorescence in the brain notably decreased, and altered swimming behavior tended to normalize. 2) In adult zebrafish, Bergenin administration reduced BMI and blood glucose levels, altered swimming behavior to slower speeds and more regular trajectories, enhanced recognition ability, decreased brain glucose and lactate levels, weakened glycolytic enzyme activities, improved pathological changes in the telencephalon and gills, reduced expression of pro-inflammatory cytokines, decreased ins expression and increased expression of irs1, irs2a, and irs2b, suggesting a reduction in insulin resistance. It also altered the expression of pparg and rela. 3) In BV2 cell line, Bergenin significantly reduced the protein expression of glycolytic enzymes (GLUT1, HK2, PKFKB3, and PKM2), lowered IL-1β, IL-6, and TNF-α mRNA expression, elevated PPAR-γ protein expression, and decreased P-NF-κB-p65 protein expression. 4) In the rat model, Bergenin improves learning and memory abilities in STZ-induced rats, mitigates neuronal damage in the hippocampal region, and reduces the expression of inflammatory factors IL-1β, IL-6, and TNF-α. Bergenin decreases brain glucose and lactate levels, as well as glycolytic enzyme activity. Furthermore, Bergenin increases PPARγ expression and decreases p-NF-κB p65/NF-κB p65 expression in the hippocampus.
Conclusion: Bergenin intervenes through the PPAR-γ/NF-κB pathway, redirecting glucose metabolism, alleviating inflammation, and preventing high glucose-induced neuronal damage.
Keywords: Zebrafish; bergenin; diabetes-associated cognitive impairment (DACI); glycolysis; neuroinflammation.
Copyright © 2024 Yu, Luo, He, Li, Yang, Zhou, He, Chen, Song and Cheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

