Timed sulfonylurea modulation improves locomotor and sensory dysfunction following spinal cord injury
- PMID: 39148545
- PMCID: PMC11324438
- DOI: 10.3389/fphar.2024.1440198
Timed sulfonylurea modulation improves locomotor and sensory dysfunction following spinal cord injury
Abstract
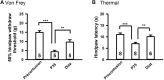
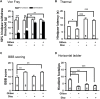
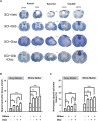
Traumatic spinal cord injury (SCI) results in immediate tissue necrosis and delayed secondary expansion of neurological damage, often resulting in lifelong paralysis, neurosensory dysfunction, and chronic pain. Progressive hemorrhagic necrosis (PHN) and excessive excitation are the main sources of secondary neural injury. Recent approaches to attenuate PHN by glibenclamide can improve locomotor function after SCI. However, use of glibenclamide can exacerbate development of SCI-induced chronic pain by inhibiting KATP channels to increase neuronal excitation and glial activation. In this study, we explored a treatment strategy involving administration of glibenclamide, which suppresses PHN, and diazoxide, which protects against neuronal excitation and inflammation, at different time intervals following spinal cord contusion. Our goal was to determine whether this combined approach enhances both sensory and motor function. Contusive SCI was induced at spinal segment T10 in adult rats. We found that KATP channels opener, diazoxide, decreased the hyperexcitability of primary sensory neurons after SCI by electrophysiology. Timed application of glibenclamide and diazoxide following contusion significantly improved locomotor function and mitigated development of SCI-induced chronic pain, as shown by behavioral evidence. Finally, we found that timed application of glibenclamide and diazoxide attenuates the inflammatory activity in the spinal cord and increases the survival of spinal matters following SCI. These preclinical studies introduce a promising potential treatment strategy to address SCI-induced dysfunction.
Keywords: ATP-gated potassium channels; chronic pain; diazoxide; glibenclamide; spinal cord injury.
Copyright © 2024 Xu, Maskey, Wu and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Basso D. M., Beattie M. S., Bresnahan J. C., Anderson D. K., Faden A. I., Gruner J. A., et al. (1996). MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J. Neurotrauma 13 (7), 343–359. 10.1089/neu.1996.13.343 - DOI - PubMed
-
- Bedi S. S., Yang Q., Crook R. J., Du J., Wu Z., Fishman H. M., et al. (2010). Chronic spontaneous activity generated in the somata of primary nociceptors is associated with pain-related behavior after spinal cord injury. J. Neurosci. 30 (44), 14870–14882. 10.1523/JNEUROSCI.2428-10.2010 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

