This is a preprint.
Linoleoyl-lysophosphatidylcholine suppresses immune-related adverse events due to immune checkpoint blockade
- PMID: 39148854
- PMCID: PMC11326322
- DOI: 10.1101/2024.08.07.24310974
Linoleoyl-lysophosphatidylcholine suppresses immune-related adverse events due to immune checkpoint blockade
Abstract
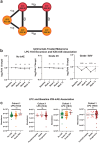
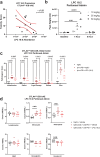
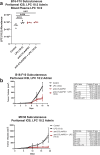
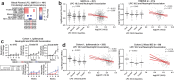
Immune related adverse events (irAEs) after immune checkpoint blockade (ICB) therapy occur in a significant proportion of cancer patients. To date, the circulating mediators of ICB-irAEs remain poorly understood. Using non-targeted mass spectrometry, here we identify the circulating bio-active lipid linoleoyl-lysophosphatidylcholine (LPC 18:2) as a modulator of ICB-irAEs. In three independent human studies of ICB treatment for solid tumor, loss of circulating LPC 18:2 preceded the development of severe irAEs across multiple organ systems. In both healthy humans and severe ICB-irAE patients, low LPC 18:2 was found to correlate with high blood neutrophilia. Reduced LPC 18:2 biosynthesis was confirmed in preclinical ICB-irAE models, and LPC 18:2 supplementation in vivo suppressed neutrophilia and tissue inflammation without impacting ICB anti-tumor response. Results indicate that circulating LPC 18:2 suppresses human ICB-irAEs, and LPC 18:2 supplementation may improve ICB outcomes by preventing severe inflammation while maintaining anti-tumor immunity.
Figures








References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
