Key quality parameter comparison of mesenchymal stem cell product cryopreserved in different cryopreservation solutions for clinical applications
- PMID: 39148941
- PMCID: PMC11324487
- DOI: 10.3389/fbioe.2024.1412811
Key quality parameter comparison of mesenchymal stem cell product cryopreserved in different cryopreservation solutions for clinical applications
Abstract
Introduction: Cryopreservation is a critical process of cell products for achieving a commercial viability through wide scale adoption. By preserving cells in a lower temperature, cryopreservation enables a product to be off-the-shelf and ready for infusion. An optimized cryopreservation strategy can maintain the viability, phenotype, and potency of thawed mesenchymal stromal/stem cells (MSCs) while being regulatory compliant. We compared three clinical-ready formulations with one research cryopreservation solutions and evaluated key quality parameters of post thawed MSCs.
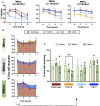
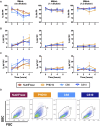
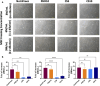
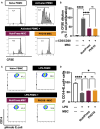
Method and result: MSCs were cryopreserved at 3, 6, and 9 million cells/mL (M/mL) in four different cryopreservation solutions: NutriFreez (10% dimethyl sulfoxide [DMSO]), Plasmalyte A (PLA)/5% human albumin (HA)/10% DMSO (PHD10), CryoStor CS5 (5% DMSO), and CryoStor CS10 (10% DMSO). To establish post thaw viability, cells were evaluated with no dilution of DMSO (from 3 M/mL), 1:1 dilution (from 6 M/mL), or 1:2 dilution (from 9 M/mL) with PLA/5% HA, to achieve uniform concentration at 3 M/mL. Cell viability was measured at 0-, 2-, 4-, and 6-h post thaw with Trypan blue exclusion and Annexin V/PI staining. Dilution (1:2) of final cell products from 9M/mL resulted in an improvement of cell viability over 6 h but showed a trend of decreased recovery. MSCs cryopreserved in solutions with 10% DMSO displayed comparable viabilities and recoveries up to 6 h after thawing, whereas a decreasing trend was noted in cell viability and recovery with CS5. Cells from all groups exhibited surface marker characteristics of MSCs. We further evaluated cell proliferation after 6-day recovery in culture. While cells cryopreserved in NutriFreez and PHD10 presented similar cell growth post thaw, MSCs cryopreserved in CS5 and CS10 at 3 M/mL and 6M/mL showed 10-fold less proliferative capacity. No significant differences were observed between MSCs cryopreserved in NutriFreez and PHD10 in their potency to inhibit T cell proliferation and improve monocytic phagocytosis.
Conclusion: MSCs can be cryopreserved up to 9 M/mL without losing notable viability and recovery, while exhibiting comparable post thaw potency with NutriFreez and PHD10. These results highlight the importance of key parameter testing for selecting the optimal cryopreservation solution for MSC-based therapy.
Keywords: cell therapy; cryopreservation; final cell products; mesenchymal stem cells; off-the-shelf; potency; quality; stability.
Copyright © 2024 Tan, Salkhordeh, Murray, Souza-Moreira, Stewart and Mei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Abramson J. S., Palomba M. L., Gordon L. I., Lunning M. A., Wang M., Arnason J., et al. (2020). Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 396 (10254), 839–852. 10.1016/s0140-6736(20)31366-0 - DOI - PubMed
-
- Acker J. P. (2005). “Biopreservation of cells and engineered tissues,” in Tissue engineering II, Editors Lee K., Kaplan D. (Berlin Heidelberg: Springer; ), 157–187. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

