Thiazolidine-2,4-dione hybrids as dual alpha-amylase and alpha-glucosidase inhibitors: design, synthesis, in vitro and in vivo anti-diabetic evaluation
- PMID: 39149094
- PMCID: PMC11324062
- DOI: 10.1039/d4md00199k
Thiazolidine-2,4-dione hybrids as dual alpha-amylase and alpha-glucosidase inhibitors: design, synthesis, in vitro and in vivo anti-diabetic evaluation
Abstract
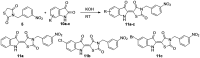
Twelve 3,5-disubstituted-thiazolidine-2,4-dione (TZD) hybrids were synthesized using solution phase chemistry. Continuing our previous work, nine O-modified ethyl vanillin (8a-i) derivatives were synthesized and reacted with the TZD core via Knoevenagel condensation under primary reaction conditions to obtain final derivatives 9a-i. Additionally, three isatin-TZD hybrids (11a-c) were synthesized. The intermediates and final derivatives were characterized using 1H and 13C NMR spectroscopy, and the observed chemical shifts agreed with the proposed structures. The in vitro alpha-amylase and alpha-glucosidase inhibitory evaluation of newly synthesized derivatives revealed compounds 9F and 9G as the best dual inhibitors, with IC50 values of 9.8 ± 0.047 μM for alpha-glucosidase (9F) and 5.15 ± 0.0017 μM for alpha-glucosidase (9G), 17.10 ± 0.015 μM for alpha-amylase (9F), and 9.2 ± 0.092 μM for alpha-amylase (9G). The docking analysis of synthesized compounds indicated that compounds have a higher binding affinity for alpha-glucosidase as compared to alpha-amylase, as seen from docking scores ranging from -1.202 to -5.467 (for alpha-amylase) and -4.373 to -7.300 (for alpha-glucosidase). Further, the molecules possess a high LD50 value, typically ranging from 1000 to 1600 mg kg-1 of body weight, and exhibit non-toxic properties. The in vitro cytotoxicity assay results on PANC-1 and INS-1 cells demonstrated that the compounds were devoid of significant toxicity against the tested cells. Compounds 9F and 9G showed high oral absorption, i.e., oral absorption >96%, and their molecular dynamics simulation yielded results closely aligned with the observed docking outcomes. Finally, compounds 9F and 9G were evaluated for in vivo antidiabetic assessment by the induction of diabetes in Wistar rats using streptozotocin. Molecule 9G has been identified as the most effective anti-diabetic molecule due to its ability to modulate several biochemical markers in blood plasma and tissue homogenates. The results were further confirmed by histology investigations conducted on isolated pancreas, liver, and kidney.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures































References
-
- Zhao X. Zhang Y. Yang Y. Pan J. Diabetes-related avoidable hospitalisations and its relationship with primary healthcare resourcing in China: A cross-sectional study from Sichuan Province. Health Soc. Care Community. 2022;30(4):e1143–e1156. - PubMed
-
- Li J.-M. Li X. Chan L. W. Hu R. Zheng T. Li H. et al., Lipotoxicity-polarised macrophage-derived exosomes regulate mitochondrial fitness through Miro1-mediated mitophagy inhibition and contribute to type 2 diabetes development in mice. Diabetologia. 2023;66(12):2368–2386. doi: 10.1007/s00125-023-05992-7. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

