Antitubercular evaluation of dihydropyridine-triazole conjugates: design, synthesis, in vitro screening, SAR and in silico ADME predictions
- PMID: 39149103
- PMCID: PMC11324066
- DOI: 10.1039/d4md00377b
Antitubercular evaluation of dihydropyridine-triazole conjugates: design, synthesis, in vitro screening, SAR and in silico ADME predictions
Abstract
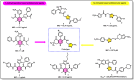
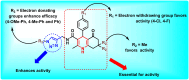
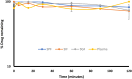
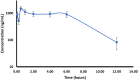
This study investigates the potential of click chemistry for the development of novel anti-tuberculosis agents. A targeted library of 1,4-dihydropyridine-1,2,3-triazole conjugates was synthesized and evaluated for their in vitro activity against Mycobacterium tuberculosis H37Ra using the resazurin microtiter assay (REMA). Among the synthesized derivatives, compounds J10, J11, J14, J22 and J23 demonstrated significant antimycobacterial activity. These compounds exhibited low MIC values ranging from 6.24 to 6.64 μg mL-1, highlighting their promising potential as lead compounds for further developing novel tuberculosis therapeutics. In addition to the promising in vitro activity, structure-activity relationship (SAR) analysis revealed that electron-withdrawing groups on the aryl-substituted ring of the dihydropyridines (J10-J24), a triazole with an unsubstituted aryl ring or with electron-donating groups (methyl or methoxy), and a geminal dimethyl group are essential structural features for the observed antitubercular activity. Furthermore, in silico ADME (absorption, distribution, metabolism, and excretion) parameters and pharmacokinetic studies supported the potential of these conjugates for oral bioavailability. These findings collectively highlight the 1,4-dihydropyridine-1,2,3-triazole scaffold as a promising platform for developing novel orally active anti-tuberculosis drugs.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Prabhu R. Singh V. Adv. Microbiol. 2019;9:931. doi: 10.4236/aim.2019.911059. - DOI
-
- https://www.who.int/campaigns/world-tb-day/2024, (accessed 24 March 2024)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

