Non-coding RNAs as therapeutic targets in cancer and its clinical application
- PMID: 39149142
- PMCID: PMC11325817
- DOI: 10.1016/j.jpha.2024.02.001
Non-coding RNAs as therapeutic targets in cancer and its clinical application
Abstract
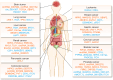
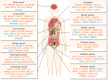
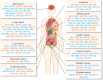
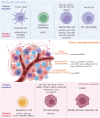
Cancer genomics has led to the discovery of numerous oncogenes and tumor suppressor genes that play critical roles in cancer development and progression. Oncogenes promote cell growth and proliferation, whereas tumor suppressor genes inhibit cell growth and division. The dysregulation of these genes can lead to the development of cancer. Recent studies have focused on non-coding RNAs (ncRNAs), including circular RNA (circRNA), long non-coding RNA (lncRNA), and microRNA (miRNA), as therapeutic targets for cancer. In this article, we discuss the oncogenes and tumor suppressor genes of ncRNAs associated with different types of cancer and their potential as therapeutic targets. Here, we highlight the mechanisms of action of these genes and their clinical applications in cancer treatment. Understanding the molecular mechanisms underlying cancer development and identifying specific therapeutic targets are essential steps towards the development of effective cancer treatments.
Keywords: Cancer; Clinical application; Non-coding RNA; Therapeutic targets.
© 2024 The Author(s).
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

