This is a preprint.
Integrated Single-Cell Multiomic Profiling of Caudate Nucleus Suggests Key Mechanisms in Alcohol Use Disorder
- PMID: 39149227
- PMCID: PMC11326171
- DOI: 10.1101/2024.08.02.606355
Integrated Single-Cell Multiomic Profiling of Caudate Nucleus Suggests Key Mechanisms in Alcohol Use Disorder
Update in
-
Integrated single-cell multiomic profiling of caudate nucleus suggests key mechanisms in alcohol use disorder.Nat Commun. 2025 Oct 13;16(1):9070. doi: 10.1038/s41467-025-64136-0. Nat Commun. 2025. PMID: 41083468 Free PMC article.
Abstract
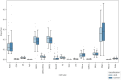
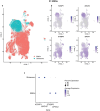
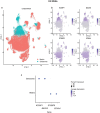
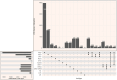
Alcohol use disorder (AUD) induces complex transcriptional and regulatory changes across multiple brain regions including the caudate nucleus, which remains understudied. Using paired single-nucleus RNA-seq and ATAC-seq on caudate samples from 143 human postmortem brains, including 74 with AUD, we identified 17 distinct cell types. We found that a significant portion of the alcohol-induced changes in gene expression occurred through altered chromatin accessibility. Notably, we identified novel transcriptional and chromatin accessibility differences in medium spiny neurons, impacting pathways such as RNA metabolism and immune response. A small cluster of D1/D2 hybrid neurons showed distinct differences, suggesting a unique role in AUD. Microglia exhibited distinct activation states deviating from classical M1/M2 designations, and astrocytes entered a reactive state partially regulated by JUND, affecting glutamatergic synapse pathways. Oligodendrocyte dysregulation, driven in part by OLIG2, was linked to demyelination and increased TGF-β1 signaling from microglia and astrocytes. We also observed increased microglia-astrocyte communication via the IL-1β pathway. Leveraging our multiomic data, we performed cell type-specific expression quantitative trait loci analysis, integrating that with public genome-wide association studies to identify AUD risk genes such as ADAL and PPP2R3C, providing a direct link between genetic variants, chromatin accessibility, and gene expression in AUD. These findings not only provide new insights into the genetic and cellular mechanisms in the caudate related to AUD but also demonstrate the broader utility of large-scale multiomic studies in uncovering complex gene regulation across diverse cell types, which has implications beyond the substance use field.
Figures








References
-
- Organization, W. H. Global Status Report on Alcohol and Health 2018. (World Health Organization, 2018).
-
- Diagnostic and statistical manual of mental disorders : DSM-5™. 5th edition. edn, (American Psychiatric Publishing, a division of American Psychiatric Association, 2013).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
