This is a preprint.
Outer hair cells stir cochlear fluids
- PMID: 39149246
- PMCID: PMC11326228
- DOI: 10.1101/2024.08.07.607009
Outer hair cells stir cochlear fluids
Update in
-
Outer hair cells stir cochlear fluids.Elife. 2025 Jan 16;13:RP101943. doi: 10.7554/eLife.101943. Elife. 2025. PMID: 39817719 Free PMC article.
Abstract
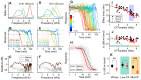
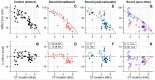
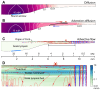
We hypothesized that active outer hair cells drive cochlear fluid circulation. The hypothesis was tested by delivering the neurotoxin, kainic acid, to the intact round window of young gerbil cochleae while monitoring auditory responses in the cochlear nucleus. Sounds presented at a modest level significantly expedited kainic acid delivery. When outer-hair-cell motility was suppressed by salicylate, the facilitation effect was compromised. A low-frequency tone was more effective than broadband noise, especially for drug delivery to apical locations. Computational model simulations provided the physical basis for our observation, which incorporated solute diffusion, fluid advection, fluid-structure interaction, and outer-hair-cell motility. Active outer hair cells deformed the organ of Corti like a peristaltic tube to generate apically streaming flows along the tunnel of Corti and basally streaming flows along the scala tympani. Our measurements and simulations coherently suggest that active outer hair cells in the tail region of cochlear traveling waves drive cochlear fluid circulation.
Keywords: drug delivery; electromotility; kainic acid; organ of Corti; outer hair cell; perilymph.
Figures


References
-
- Berg H. C. (1993). Random walks in biology. Princeton, N.J., Princeton University Press.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
