This is a preprint.
TBK1 and IKKε protect target cells from IFNγ-mediated T cell killing via an inflammatory apoptotic mechanism
- PMID: 39149268
- PMCID: PMC11326184
- DOI: 10.1101/2024.08.06.606693
TBK1 and IKKε protect target cells from IFNγ-mediated T cell killing via an inflammatory apoptotic mechanism
Abstract
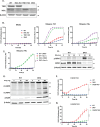
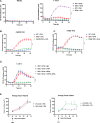
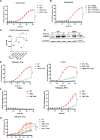
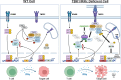
Cytotoxic T cells produce interferon gamma (IFNγ), which plays a critical role in anti-microbial and anti-tumor responses. However, it is not clear whether T cell-derived IFNγ directly kills infected and tumor target cells, and how this may be regulated. Here, we report that target cell expression of the kinases TBK1 and IKKε regulate IFNγ cytotoxicity by suppressing the ability of T cell-derived IFNγ to kill target cells. In tumor targets lacking TBK1 and IKKε, IFNγ induces expression of TNFR1 and the Z-nucleic acid sensor, ZBP1, to trigger RIPK1-dependent apoptosis, largely in a target cell-autonomous manner. Unexpectedly, IFNγ, which is not known to signal to NFκB, induces hyperactivation of NFκB in TBK1 and IKKε double-deficient cells. TBK1 and IKKε suppress IKKα/β activity and in their absence, IFNγ induces elevated NFκB-dependent expression of inflammatory chemokines and cytokines. Apoptosis is thought to be non-inflammatory, but our observations demonstrate that IFNγ can induce an inflammatory form of apoptosis, and this is suppressed by TBK1 and IKKε. The two kinases provide a critical connection between innate and adaptive immunological responses by regulating three key responses: (1) phosphorylation of IRF3/7 to induce type I IFN; (2) inhibition of RIPK1-dependent death; and (3) inhibition of NFκB-dependent inflammation. We propose that these kinases evolved these functions such that their inhibition by pathogens attempting to block type I IFN expression would enable IFNγ to trigger apoptosis accompanied by an alternative inflammatory response. Our findings show that loss of TBK1 and IKKε in target cells sensitizes them to inflammatory apoptosis induced by T cell-derived IFNγ.
Conflict of interest statement
Conflict of Interest We declare that there are no financial conflicts of interest.
Figures


References
-
- Maraskovsky E., Chen W.F. & Shortman K. IL-2 and IFN-gamma are two necessary lymphokines in the development of cytolytic T cells. J Immunol 143, 1210–1214 (1989). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
