This is a preprint.
The molecular basis of Human FN3K mediated phosphorylation of glycated substrate
- PMID: 39149269
- PMCID: PMC11326186
- DOI: 10.1101/2024.08.05.606604
The molecular basis of Human FN3K mediated phosphorylation of glycated substrate
Update in
-
The molecular basis of Human FN3K mediated phosphorylation of glycated substrates.Nat Commun. 2025 Jan 22;16(1):941. doi: 10.1038/s41467-025-56207-z. Nat Commun. 2025. PMID: 39843453 Free PMC article.
Abstract
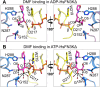
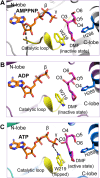
Glycation, a non-enzymatic post-translational modification occurring on proteins, can be actively reversed via site-specific phosphorylation of the fructose-lysine moiety by FN3K kinase, to impact the cellular function of target protein. A regulatory axis between FN3K and glycated protein targets has been associated with conditions like diabetes and cancer. However the molecular basis of this relationship has not been explored so far. Here, we determined a series of crystal structures of HsFN3K in apo-state, and in complex with different nucleotide analogs together with a sugar substrate mimic to reveal the features important for its kinase activity and substrate recognition. Additionally, the dynamics in sugar substrate binding during the kinase catalytic cycle provide important mechanistic insights into HsFN3K function. Our structural work provides the molecular basis for rationale small molecule design targeting FN3K.
Conflict of interest statement
Declaration of interest Authors declare no competing financial interest.
Figures





Similar articles
-
The molecular basis of Human FN3K mediated phosphorylation of glycated substrates.Nat Commun. 2025 Jan 22;16(1):941. doi: 10.1038/s41467-025-56207-z. Nat Commun. 2025. PMID: 39843453 Free PMC article.
-
Increased protein glycation in fructosamine 3-kinase-deficient mice.Biochem J. 2006 Oct 15;399(2):257-64. doi: 10.1042/BJ20060684. Biochem J. 2006. PMID: 16819943 Free PMC article.
-
Fructosamine 3-kinase-related protein and deglycation in human erythrocytes.Biochem J. 2004 Aug 15;382(Pt 1):137-43. doi: 10.1042/BJ20040307. Biochem J. 2004. PMID: 15137908 Free PMC article.
-
The Taming of Nuclear Factor Erythroid-2-Related Factor-2 (Nrf2) Deglycation by Fructosamine-3-Kinase (FN3K)-Inhibitors-A Novel Strategy to Combat Cancers.Cancers (Basel). 2021 Jan 14;13(2):281. doi: 10.3390/cancers13020281. Cancers (Basel). 2021. PMID: 33466626 Free PMC article. Review.
-
Dietary glycation compounds - implications for human health.Crit Rev Toxicol. 2024 Sep;54(8):485-617. doi: 10.1080/10408444.2024.2362985. Epub 2024 Aug 16. Crit Rev Toxicol. 2024. PMID: 39150724
References
-
- Hodge J.E. (1955) The Amadori rearrangement. Adv Carbohydr Chem, 10, 169–205. - PubMed
-
- Baynes J.W., Watkins N.G., Fisher C.I., Hull C.J., Patrick J.S., Ahmed M.U., Dunn J.A. and Thorpe S.R. (1989) The Amadori product on protein: structure and reactions. Prog Clin Biol Res, 304, 43–67. - PubMed
-
- Brownlee M. (1991) Glycosylation products as toxic mediators of diabetic complications. Annu Rev Med, 42, 159–166. - PubMed
-
- Ahmed M.U., Thorpe S.R. and Baynes J.W. (1986) Identification of N epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated protein. J Biol Chem, 261, 4889–4894. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
