This is a preprint.
Investigating cocaine- and abstinence-induced effects on astrocyte gene expression in the nucleus accumbens
- PMID: 39149305
- PMCID: PMC11326167
- DOI: 10.1101/2024.08.05.606656
Investigating cocaine- and abstinence-induced effects on astrocyte gene expression in the nucleus accumbens
Abstract
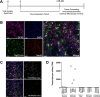
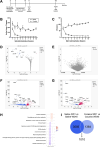
In recent years, astrocytes have been increasingly implicated in cellular mechanisms of substance use disorders (SUD). Astrocytes are structurally altered following exposure to drugs of abuse; specifically, astrocytes within the nucleus accumbens (NAc) exhibit significantly decreased surface area, volume, and synaptic colocalization after operant self-administration of cocaine and extinction or protracted abstinence (45 days). However, the mechanisms that elicit these morphological modifications are unknown. The current study aims to elucidate the molecular modifications that lead to observed astrocyte structural changes in rats across cocaine abstinence using astrocyte-specific RiboTag and RNA-seq, as an unbiased, comprehensive approach to identify genes whose transcription or translation change within NAc astrocytes following cocaine self-administration and extended abstinence. Using this method, our data reveal cellular processes including cholesterol biosynthesis that are altered specifically by cocaine self-administration and abstinence, suggesting that astrocyte involvement in these processes is changed in cocaine-abstinent rats. Overall, the results of this study provide insight into astrocyte functional adaptations that occur due to cocaine exposure or during cocaine withdrawal, which may pinpoint further mechanisms that contribute to cocaine-seeking behavior.
Keywords: Abstinence; Astrocyte; Cocaine; Nucleus Accumbens; RNA Sequencing; RiboTag.
Figures

References
-
- Araque A., & Perea G. (2004). Glial modulation of synaptic transmission in culture. Glia, 47(3), 241–248. - PubMed
-
- Batiuk M. Y., Martirosyan A., Wahis J., de Vin F., Marneffe C., Kusserow C., Koeppen J., Viana J. F., Oliveira J. F., Voet T., Ponting C. P., Belgard T. G., & Holt M. G. (2020). Identification of region-specific astrocyte subtypes at single cell resolution. Nature communications, 11(1), 1220.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
