This is a preprint.
The synaptonemal complex aligns meiotic chromosomes by wetting
- PMID: 39149313
- PMCID: PMC11326210
- DOI: 10.1101/2024.08.07.607092
The synaptonemal complex aligns meiotic chromosomes by wetting
Update in
-
The synaptonemal complex aligns meiotic chromosomes by wetting.Sci Adv. 2025 Feb 28;11(9):eadt5675. doi: 10.1126/sciadv.adt5675. Epub 2025 Feb 26. Sci Adv. 2025. PMID: 40009663 Free PMC article.
Abstract
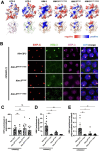
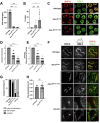
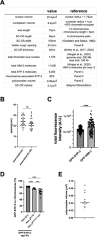
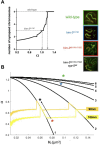
During meiosis, the parental chromosomes are drawn together to enable exchange of genetic information. Chromosomes are aligned through the assembly of a conserved interface, the synaptonemal complex, composed of a central region that forms between two parallel chromosomal backbones called axes. Here we identify the axis-central region interface in C. elegans, containing a conserved positive patch on the axis component HIM-3 and the C-terminus of the central region protein SYP-5. Crucially, the canonical ultrastructure of the synaptonemal complex is altered upon weakening this interface. We developed a thermodynamic model that recapitulates our experimental observations, indicating that the liquid-like central region can assemble by wetting the axes without active energy consumption. More broadly, our data show that condensation drives tightly regulated nuclear reorganization during sexual reproduction.
Keywords: C. elegans; HORMA; condensation; meiosis; synaptonemal complex; wetting.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Brangwynne C. P., Eckmann C. R., Courson D. S., Rybarska A., Hoege C., et al. , 2009. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324:1729–1732. - PubMed
-
- Chung Y.-C., and Tu L.-C., 2023. Interplay of dynamic genome organization and biomolecular condensates. Curr. Opin. Cell Biol. 85:102252. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
