This is a preprint.
Arsenic impairs Drosophila neural stem cell mitotic progression and sleep behavior in a tauopathy model
- PMID: 39149321
- PMCID: PMC11326188
- DOI: 10.1101/2024.08.05.606375
Arsenic impairs Drosophila neural stem cell mitotic progression and sleep behavior in a tauopathy model
Update in
-
Arsenic impairs Drosophila neural stem cell mitotic progression and sleep behavior in a tauopathy model.G3 (Bethesda). 2025 May 8;15(5):jkaf049. doi: 10.1093/g3journal/jkaf049. G3 (Bethesda). 2025. PMID: 40192438 Free PMC article.
Abstract
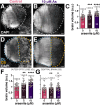
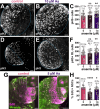
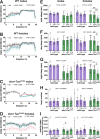
Despite established exposure limits, arsenic remains the most significant environmental risk factor detrimental to human health and is associated with carcinogenesis and neurotoxicity. Arsenic compromises neurodevelopment, and it is associated with peripheral neuropathy in adults. Exposure to heavy metals, such as arsenic, may also increase the risk of neurodegenerative disorders. Nevertheless, the molecular mechanisms underlying arsenic-induced neurotoxicity remain poorly understood. Elucidating how arsenic contributes to neurotoxicity may mitigate some of the risks associated with chronic sublethal exposure and inform future interventions. In this study, we examine the effects of arsenic exposure on Drosophila larval neurodevelopment and adult neurologic function. Consistent with prior work, we identify significant developmental delays and heightened mortality in response to arsenic. Within the developing larval brain, we identify a dose-dependent increase in brain volume. This aberrant brain growth is coupled with impaired mitotic progression of the neural stem cells (NSCs), progenitors of the neurons and glia of the central nervous system. Live imaging of cycling NSCs reveals significant delays in cell cycle progression upon arsenic treatment, leading to genomic instability. In adults, chronic arsenic exposure reduces neurologic function, such as locomotion. Finally, we show arsenic selectively impairs circadian rhythms in a humanized tauopathy model. These findings inform mechanisms of arsenic neurotoxicity and reveal sex-specific and genetic vulnerabilities to sublethal exposure.
Keywords: arsenic; genome instability; neural stem cells; neurodevelopment; neurotoxicity.
Conflict of interest statement
Competing interests The authors have no competing interests to declare.
Figures



References
-
- ATSDR. Arsenic Toxicity 2009. [updated May 19, 2023]. Available from: https://www.atsdr.cdc.gov/csem/arsenic/what_routes.html.
-
- WHO. Arsenic 2022. [cited 2024]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/arsenic.
-
- Rodriguez-Barranco M, Lacasana M, Aguilar-Garduno C, Alguacil J, Gil F, Gonzalez-Alzaga B, Rojas-Garcia A. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. Sci Total Environ. 2013;454–455:562–77. Epub 20130409. doi: 10.1016/j.scitotenv.2013.03.047. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
