This is a preprint.
Drug Metabolism and Transport Capacity of Endothelial Cells, Pericytes, and Astrocytes: Implications for CNS Drug Disposition
- PMID: 39149336
- PMCID: PMC11326144
- DOI: 10.1101/2024.08.01.606165
Drug Metabolism and Transport Capacity of Endothelial Cells, Pericytes, and Astrocytes: Implications for CNS Drug Disposition
Abstract
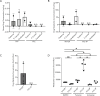
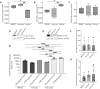
Therapeutically targeting the brain requires interactions with endothelial cells, pericytes, and astrocytes at the blood brain barrier (BBB). We evaluated regional and cell-type specific drug metabolism and transport mechanisms using rhesus macaques and in vitro treatment of primary human cells. Here, we report heterogenous distribution of representative drugs, tenofovir (TFV), emtricitabine (FTC), and their active metabolites, which cerebrospinal fluid measures could not reflect. We found that all BBB cell types possessed functional drug metabolizing enzymes and transporters that promoted TFV and FTC uptake and pharmacologic activation. Pericytes and astrocytes emerged as pharmacologically dynamic cells that rivaled hepatocytes and were uniquely susceptible to modulation by disease and treatment. Together, our findings demonstrate the importance of considering the BBB as a unique pharmacologic entity, rather than viewing it as an extension of the liver, as each cell type possesses distinct drug metabolism and transport capacities that contribute to differential brain drug disposition.
Keywords: Antiretroviral therapy; Blood brain barrier; Central nervous system; Drug disposition; Drug metabolism; Drug transporter; HIV.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Chaillon A., Gianella S., Dellicour S., Rawlings S.A., Schlub T.E., Oliveira M.F.D., Ignacio C., Porrachia M., Vrancken B., and Smith D.M. (2020). HIV persists throughout deep tissues with repopulation from multiple anatomical sources. J. Clin. Investig. 130, 1699–1712. 10.1172/jci134815. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
