This is a preprint.
DMT1 knockout abolishes ferroptosis induced mitochondrial dysfunction in C. elegans amyloid β proteotoxicity
- PMID: 39149382
- PMCID: PMC11326247
- DOI: 10.1101/2024.08.08.607074
DMT1 knockout abolishes ferroptosis induced mitochondrial dysfunction in C. elegans amyloid β proteotoxicity
Update in
-
DMT1 knockout abolishes ferroptosis induced mitochondrial dysfunction in C. elegans amyloid β proteotoxicity.Free Radic Biol Med. 2024 Nov 1;224:785-796. doi: 10.1016/j.freeradbiomed.2024.09.034. Epub 2024 Sep 22. Free Radic Biol Med. 2024. PMID: 39317269
Abstract
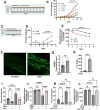
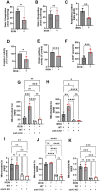
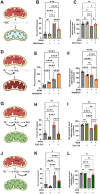
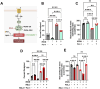
Iron is critical for neuronal activity and metabolism, and iron dysregulation alters these functions in age-related neurodegenerative disorders, such as Alzheimer's disease (AD). AD is a chronic neurodegenerative disease characterized by progressive neuronal dysfunction, memory loss and decreased cognitive function. AD patients exhibit elevated iron levels in the brain compared to age-matched non-AD individuals. However, the degree to which iron overload contributes to AD pathogenesis is unclear. Here, we evaluated the involvement of ferroptosis, an iron-dependent cell death process, in mediating AD-like pathologies in C. elegans. Results showed that iron accumulation occurred prior to the loss of neuronal function as worms age. In addition, energetic imbalance was an early event in iron-induced loss of neuronal function. Furthermore, the loss of neuronal function was, in part, due to increased mitochondrial reactive oxygen species mediated oxidative damage, ultimately resulting in ferroptotic cell death. The mitochondrial redox environment and ferroptosis were modulated by pharmacologic processes that exacerbate or abolish iron accumulation both in wild-type worms and worms with increased levels of neuronal amyloid beta (Aβ). However, neuronal Aβ worms were more sensitive to ferroptosis-mediated neuronal loss, and this increased toxicity was ameliorated by limiting the uptake of ferrous iron through knockout of divalent metal transporter 1 (DMT1). In addition, DMT1 knockout completely suppressed phenotypic measures of Aβ toxicity with age. Overall, our findings suggest that iron-induced ferroptosis alters the mitochondrial redox environment to drive oxidative damage when neuronal Aβ is overexpressed. DMT1 knockout abolishes neuronal Aβ-associated pathologies by reducing neuronal iron uptake.
Keywords: Aβ proteotoxicity; Bioenergetics; Divalent metal transporter 1; Ferroptosis; Oxidative stress.
Conflict of interest statement
Declaration of conflict of interest All the authors have no conflict of interest to report
Figures

References
-
- Crichton R.R., Dexter D.T., and Ward R.J., Brain iron metabolism and its perturbation in neurological diseases. J Neural Transm (Vienna), 2011. 118(3): p. 301–14. - PubMed
-
- Belaidi A.A. and Bush A.I., Iron neurochemistry in Alzheimer's disease and Parkinson's disease: targets for therapeutics. J Neurochem, 2016. 139 Suppl 1: p. 179–197. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
