This is a preprint.
The pseudoenzyme β-amylase9 from Arabidopsis binds to and enhances the activity of α-amylase3: A possible mechanism to promote stress-induced starch degradation
- PMID: 39149391
- PMCID: PMC11326238
- DOI: 10.1101/2024.08.07.607052
The pseudoenzyme β-amylase9 from Arabidopsis binds to and enhances the activity of α-amylase3: A possible mechanism to promote stress-induced starch degradation
Update in
-
The Pseudoenzyme β-Amylase9 From Arabidopsis Activates α-Amylase3: A Possible Mechanism to Promote Stress-Induced Starch Degradation.Proteins. 2025 Jun;93(6):1189-1201. doi: 10.1002/prot.26803. Epub 2025 Jan 23. Proteins. 2025. PMID: 39846389 Free PMC article.
Abstract
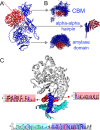
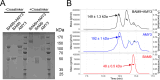
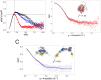
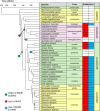
Starch accumulation in plant tissues provides an important carbon source at night and for regrowth after periods of dormancy and in times of stress. Both ɑ- and β-amylases (AMYs and BAMs, respectively) catalyze starch hydrolysis, but their functional roles are unclear. Moreover, the presence of catalytically inactive amylases that show starch excess phenotypes when deleted presents an interesting series of questions on how starch degradation is regulated. Plants lacking one of these catalytically inactive β-amylases, BAM9, were shown to have enhanced starch accumulation when combined with mutations in BAM1 and BAM3, the primary starch degrading BAMs in response to stress and at night, respectively. Importantly, BAM9 has been reported to be transcriptionally induced by stress through activation of SnRK1. Using yeast two-hybrid experiments, we identified the plastid-localized AMY3 as a potential interaction partner for BAM9. We found that BAM9 interacted with AMY3 in vitro and that BAM9 enhances AMY3 activity 3-fold. Modeling of the AMY3-BAM9 complex revealed a previously undescribed N-terminal structural feature in AMY3 that we call the alpha-alpha hairpin that could serve as a potential interaction site. Additionally, AMY3 lacking the alpha-alpha hairpin is unaffected by BAM9. Structural analysis of AMY3 showed that it can form a homodimer in solution and that BAM9 appears to replace one of the AMY3 monomers to form a heterodimer. Collectively these data suggest that BAM9 is a pseudoamylase that activates AMY3 in response to cellular stress, possibly facilitating starch degradation to provide an additional energy source for stress recovery.
Figures


References
-
- Adachi M. et al. (1998) ‘Crystal structure of recombinant soybean beta-amylase complexed with beta-cyclodextrin’, The Journal of biological chemistry, 273(31), pp. 19859–19865. - PubMed
-
- Baena-González E. et al. (2007) ‘A central integrator of transcription networks in plant stress and energy signalling’, Nature, 448(7156), pp. 938–942. - PubMed
-
- Chandler J.W., Apel K. and Melzer S. (2001) ‘A novel putative β-amylase gene and ATβ-Amy from Arabidopsis thaliana are circadian regulated’, Plant science: an international journal of experimental plant biology, 161(5), pp. 1019–1024.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
