This is a preprint.
Cysteine depletion triggers adipose tissue thermogenesis and weight-loss
- PMID: 39149397
- PMCID: PMC11326254
- DOI: 10.1101/2024.08.06.606880
Cysteine depletion triggers adipose tissue thermogenesis and weight-loss
Update in
-
Cysteine depletion triggers adipose tissue thermogenesis and weight loss.Nat Metab. 2025 Jun;7(6):1204-1222. doi: 10.1038/s42255-025-01297-8. Epub 2025 Jun 3. Nat Metab. 2025. PMID: 40461845 Free PMC article.
Abstract
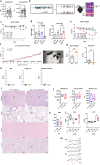
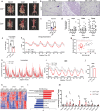
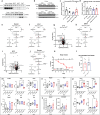
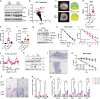
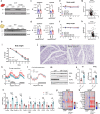
Dietary interventions such as caloric restriction (CR)1 and methionine restriction2 that prolong lifespan induce the 'browning' of white adipose tissue (WAT), an adaptive metabolic response that increases heat production to maintain health3,4. However, how diet influences adipose browning and metabolic health is unclear. Here, we identified that weight-loss induced by CR in humans5 reduces cysteine concentration in WAT suggesting depletion of this amino-acid may be involved in metabolic benefits of CR. To investigate the role of cysteine on organismal metabolism, we created a cysteine-deficiency mouse model in which dietary cysteine was eliminated and cystathionine γ-lyase (CTH)6, the enzyme that synthesizes cysteine was conditionally deleted. Using this animal model, we found that systemic cysteine-depletion causes drastic weight-loss with increased fat utilization and browning of adipose tissue. The restoration of dietary cysteine in cysteine-deficient mice rescued weight loss together with reversal of adipose browning and increased food-intake in an on-demand fashion. Mechanistically, cysteine deficiency induced browning and weight loss is dependent on sympathetic nervous system derived noradrenaline signaling via β3-adrenergic-receptors and does not require UCP1. Therapeutically, in high-fat diet fed obese mice, one week of cysteine-deficiency caused 30% weight-loss and reversed inflammation. These findings thus establish that cysteine is essential for organismal metabolism as removal of cysteine in the host triggers adipose browning and rapid weight loss.
Figures






References
-
- Fabbiano S, Suárez-Zamorano N, Rigo D, Veyrat-Durebex C, Stevanovic Dokic A, Colin DJ, Trajkovski M. Caloric Restriction Leads to Browning of White Adipose Tissue through Type 2 Immune Signaling. Cell Metab. 2016. Sep 13;24(3):434–446. - PubMed
-
- Chouchani ET, Kazak L, Spiegelman BM. New Advances in adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019. Jan 8;29(1):27–37. - PubMed
-
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004. Jan;84(1):277–359. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
