This is a preprint.
Spatiotemporal cerebral blood flow dynamics underlies emergence of the limbic-sensorimotor-association cortical gradient in human infancy
- PMID: 39149463
- PMCID: PMC11326418
- DOI: 10.21203/rs.3.rs-4761517/v1
Spatiotemporal cerebral blood flow dynamics underlies emergence of the limbic-sensorimotor-association cortical gradient in human infancy
Update in
-
Spatiotemporal cerebral blood flow dynamics underlies emergence of the limbic-sensorimotor-association cortical gradient in human infancy.Nat Commun. 2024 Oct 17;15(1):8944. doi: 10.1038/s41467-024-53354-7. Nat Commun. 2024. PMID: 39414859 Free PMC article.
Abstract
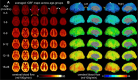
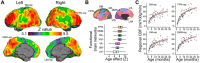
Infant cerebral blood flow (CBF) delivers nutrients and oxygen to fulfill brain energy consumption requirements for the fastest period of postnatal brain development across the lifespan. However, organizing principle of whole-brain CBF dynamics during infancy remains obscure. Leveraging a unique cohort of 100+ infants with high-resolution arterial spin labeled MRI, we found the emergence of the cortical hierarchy revealed by the highest-resolution infant CBF maps available to date. Infant CBF across cortical regions increased in a biphasic pattern with initial rapid and sequentially slower rate, with break-point ages increasing along the limbic-sensorimotor-association cortical gradient. Increases in CBF in sensorimotor cortices were associated with enhanced language and motor skills, and frontoparietal association cortices for cognitive skills. The study discovered emergence of the hierarchical limbic-sensorimotor-association cortical gradient in infancy, and offers standardized reference of infant brain CBF and insight into the physiological basis of cortical specialization and real-world infant developmental functioning.
Keywords: arterial-spin-labeled MRI; behavior; brain development; cerebral blood flow; infant; limbic-sensorimotor-association cortical gradient; neurodevelopmental outcome.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

