This is a preprint.
Bi-specific antibody engagers for cancer immunotherapy
- PMID: 39149504
- PMCID: PMC11326407
- DOI: 10.21203/rs.3.rs-4792057/v1
Bi-specific antibody engagers for cancer immunotherapy
Update in
-
Nanobody-based bispecific antibody engagers targeting CTLA-4 or PD-L1 for cancer immunotherapy.Nat Biomed Eng. 2025 Jul 16. doi: 10.1038/s41551-025-01447-z. Online ahead of print. Nat Biomed Eng. 2025. PMID: 40670719
Abstract
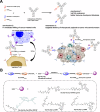
Bispecific antibody engagers are fusion proteins composed of a nanobody that recognizes immunoglobulin kappa light chains ( ) and a nanobody that recognizes either CTLA-4 or PD-L1. These fusions show strong antitumor activity in mice through recruitment of polyclonal immunoglobulins independently of specificity or isotype. In the MC38 mouse model of colorectal carcinoma, the anti-CTLA-4 conjugate eradicates tumors and reduces the number of intratumoral regulatory T cells. The anti-PD-L1 conjugate is less effective in the MC38 model, whilst still outperforming an antibody of similar specificity. The potency of the anti-PD-L1 conjugate was strongly enhanced by installation of the cytotoxic drug maytansine or a STING agonist. The ability of such fusions to engage the Fc-mediated functions of all immunoglobulin isotypes is an appealing strategy to further improve on the efficacy of immune checkpoint blockade, commonly delivered as a monoclonal immunoglobulin of a single defined isotype.
Conflict of interest statement
Declarations Competing interests X.L., and H.L.P. have filed a patent covering the technologies described in this publication (International Publication Number: WO2023141500A2). The other authors declare no competing interests. Additional Declarations: Yes there is potential Competing Interest. X.L., and H.L.P. have filed a patent covering the technologies described in this publication (International Publication Number: WO2023141500A2). The other authors declare no competing interests.
Figures






References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

