Synergistic rheumatoid arthritis therapy by interrupting the detrimental feedback loop to orchestrate hypoxia M1 macrophage polarization using an enzyme-catalyzed nanoplatform
- PMID: 39149592
- PMCID: PMC11324459
- DOI: 10.1016/j.bioactmat.2024.07.026
Synergistic rheumatoid arthritis therapy by interrupting the detrimental feedback loop to orchestrate hypoxia M1 macrophage polarization using an enzyme-catalyzed nanoplatform
Abstract
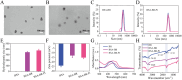
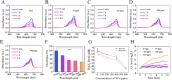
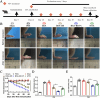
A detrimental feedback loop between hypoxia and oxidative stress consistently drives macrophage polarization toward a pro-inflammatory M1 phenotype, thus persistently aggravating rheumatoid arthritis (RA) progression. Herein, an enzyme-catalyzed nanoplatform with synergistic hypoxia-relieving and reactive oxygen species (ROS)-scavenging properties was developed using bovine serum albumin-bilirubin-platinum nanoparticles (BSA-BR-Pt NPs). Bilirubin was employed to eliminate ROS, while platinum exhibited a synergistic effect in scavenging ROS and simultaneously generated oxygen. In mice RA model, BSA-BR-Pt NPs treatment exhibited superior effects, resulting in significant improvements in joint inflammation, cartilage damage, and bone erosion, compared to methotrexate, the most widely used antirheumatic drug. Mechanistically, RNA-sequencing data and experimental results elucidated that BSA-BR-Pt NPs induced a re-polarization of hypoxic M1 macrophages to M2 macrophages via switching glycolysis to oxidative phosphorylation through the inhibition of HIF-1α pathway. Collectively, this research for the first time elaborated the underlying mechanism of enzyme-catalyzed nanoplatform in orchestrating macrophage polarization, and identified a novel therapeutic strategy for RA and other inflammatory disorders.
Keywords: Hypoxia; Macrophage; Metabolic regulation; Nanoplatform; Oxidative stress; Rheumatoid arthritis.
© 2024 The Authors.
Conflict of interest statement
The authors assert that the research was carried out without any commercial or financial affiliations that could be perceived as a possible conflict of interest. Fig. 6A is created using Figdraw (www.figdraw.com).
Figures








References
-
- Geng W., Zhao J., Tao B., Yang Y., Duan Q., Gao P., He T., Liu S., Feng Q., Zhao P., Cai K. Regulation of rheumatoid arthritis microenvironment via a self-healing injectable hydrogel for improved inflammation elimination and bone repair. Bioact. Mater. 2024;36:287–300. doi: 10.1016/j.bioactmat.2024.03.002. - DOI - PMC - PubMed
-
- Xu Y., Li Y., Gao A., Chu P.K., Wang H. Gasotransmitter delivery for bone diseases and regeneration. The Innovation Life. 2023;1(1):100015. doi: 10.59717/j.xinn-life.2023.100015. - DOI
-
- Huang R., Zhang C., Bu Y., Li Z., Zheng X., Qiu S., Machuki J.O., Zhang L., Yang Y., Guo K., Gao F. A multifunctional nano-therapeutic platform based on octahedral yolk-shell Au NR@CuS: Photothermal/photodynamic and targeted drug delivery tri-combined therapy for rheumatoid arthritis. Biomaterials. 2021;277 doi: 10.1016/j.biomaterials.2021.121088. - DOI - PubMed
LinkOut - more resources
Full Text Sources

