A functional analysis of a resorbable citrate-based composite tendon anchor
- PMID: 39149596
- PMCID: PMC11325281
- DOI: 10.1016/j.bioactmat.2024.06.030
A functional analysis of a resorbable citrate-based composite tendon anchor
Abstract
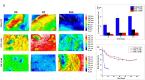
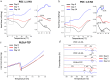
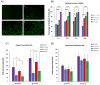
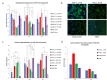
Rapid and efficient tendon fixation to a bone following trauma or in response to degenerative processes can be facilitated using a tendon anchoring device. Osteomimetic biomaterials, and in particular, bio-resorbable polymer composites designed to match the mineral phase content of native bone, have been shown to exhibit osteoinductive and osteoconductive properties in vivo and have been used in bone fixation for the past 2 decades. In this study, a resorbable, bioactive, and mechanically robust citrate-based composite formulated from poly(octamethylene citrate) (POC) and hydroxyapatite (HA) (POC-HA) was investigated as a potential tendon-fixation biomaterial. In vitro analysis with human Mesenchymal Stem Cells (hMSCs) indicated that POC-HA composite materials supported cell adhesion, growth, and proliferation and increased calcium deposition, alkaline phosphatase production, the expression of osteogenic specific genes, and activation of canonical pathways leading to osteoinduction and osteoconduction. Further, in vivo evaluation of a POC-HA tendon fixation device in a sheep metaphyseal model indicates the regenerative and remodeling potential of this citrate-based composite material. Together, this study presents a comprehensive in vitro and in vivo analysis of the functional response to a citrate-derived composite tendon anchor and indicates that citrate-based HA composites offer improved mechanical and osteogenic properties relative to commonly used resorbable tendon anchor devices formulated from poly(L-co-D, l-lactic acid) and tricalcium phosphate PLDLA-TCP.
Keywords: Citrate; Composite; Osteoinductive; Tendon anchor.
© 2024 The Authors.
Conflict of interest statement
A single researcher who participated in this study was partly funded by Acuitive Technologies Ltd. The in vivo portion of this study was funded by a research grant from Acuitive Technologies Ltd.
Figures





References
-
- Zhou Y., Hutmacher D.W., Varawan S.L., Lim T.M. In vitro bone engineering based on polycaprolactone and polycaprolactone–tricalcium phosphate composites. Polym. Int. 2007;56(3):333–342.
-
- Lohfeld S., Cahill S., Barron V., McHugh P., Dürselen L., Kreja L., Bausewein C., Ignatius A. Fabrication, mechanical and in vivo performance of polycaprolactone/tricalcium phosphate composite scaffolds. Acta Biomater. 2012;8(9):3446–3456. - PubMed
-
- Shao X., Goh J.C., Hutmacher D.W., Lee E.H., Zigang G.E. Repair of large articular osteochondral defects using hybrid scaffolds and bone marrow-derived mesenchymal stem cells in a rabbit model. Tissue Eng. 2006;12(6):1539–1551. - PubMed
-
- Fini M., Giavaresi G., Aldini N.N., Torricelli P., Botter R., Beruto D., Giardino R. A bone substitute composed of polymethylmethacrylate and α-tricalcium phosphate: results in terms of osteoblast function and bone tissue formation. Biomaterials. 2002;23(23):4523–4531. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

