Predicting Pseudomonas aeruginosa drug resistance using artificial intelligence and clinical MALDI-TOF mass spectra
- PMID: 39150244
- PMCID: PMC11406958
- DOI: 10.1128/msystems.00789-24
Predicting Pseudomonas aeruginosa drug resistance using artificial intelligence and clinical MALDI-TOF mass spectra
Abstract
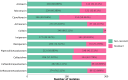
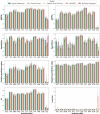
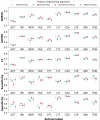
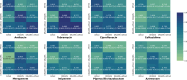
Matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) is widely used in clinical microbiology laboratories for bacterial identification but its use for detection of antimicrobial resistance (AMR) remains limited. Here, we used MALDI-TOF MS with artificial intelligence (AI) approaches to successfully predict AMR in Pseudomonas aeruginosa, a priority pathogen with complex AMR mechanisms. The highest performance was achieved for modern β-lactam/β-lactamase inhibitor drugs, namely, ceftazidime/avibactam and ceftolozane/tazobactam. For these drugs, the model demonstrated area under the receiver operating characteristic curve (AUROC) of 0.869 and 0.856, specificity of 0.925 and 0.897, and sensitivity of 0.731 and 0.714, respectively. As part of this work, we developed dynamic binning, a feature engineering technique that effectively reduces the high-dimensional feature set and has wide-ranging applicability to MALDI-TOF MS data. Compared to conventional feature engineering approaches, the dynamic binning method yielded highest performance in 7 of 10 antimicrobials. Moreover, we showcased the efficacy of transfer learning in enhancing the AUROC performance for 8 of 11 antimicrobials. By assessing the contribution of features to the model's prediction, we identified proteins that may contribute to AMR mechanisms. Our findings demonstrate the potential of combining AI with MALDI-TOF MS as a rapid AMR diagnostic tool for Pseudomonas aeruginosa.IMPORTANCEPseudomonas aeruginosa is a key bacterial pathogen that causes significant global morbidity and mortality. Antimicrobial resistance (AMR) emerges rapidly in P. aeruginosa and is driven by complex mechanisms. Drug-resistant P. aeruginosa is a major challenge in clinical settings due to limited treatment options. Early detection of AMR can guide antibiotic choices, improve patient outcomes, and avoid unnecessary antibiotic use. Matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) is widely used for rapid species identification in clinical microbiology. In this study, we repurposed mass spectra generated by MALDI-TOF and used them as inputs for artificial intelligence approaches to successfully predict AMR in P. aeruginosa for multiple key antibiotic classes. This work represents an important advance toward using MALDI-TOF as a rapid AMR diagnostic for P. aeruginosa in clinical settings.
Keywords: MALDI-TOF MS; Pseudomonas aeruginosa; antimicrobial resistance; machine learning.
Conflict of interest statement
N.M. has received research support from GlaxoSmithKline, unrelated to the current study. A.Y.P. has received research funding from MSD through an investigator-initiated research project. All other authors declare no conflict of interest.
Figures

References
-
- Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, WHO Pathogens Priority List Working Group . 2018. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18:318–327. doi: 10.1016/S1473-3099(17)30753-3 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
