RSV enhances Staphylococcus aureus bacterial growth in the lung
- PMID: 39150268
- PMCID: PMC11475690
- DOI: 10.1128/iai.00304-24
RSV enhances Staphylococcus aureus bacterial growth in the lung
Abstract
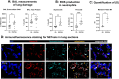
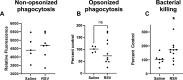
Patients coinfected with respiratory syncytial virus (RSV) and bacteria have longer hospital stays, higher risk of intensive care unit admission, and worse outcomes. We describe a model of RSV line 19F/methicillin-resistant Staphylococcus aureus (MRSA) USA300 coinfection that does not impair viral clearance, but prior RSV infection enhances USA300 MRSA bacterial growth in the lung. The increased bacterial burden post-RSV correlates with reduced accumulation of neutrophils and impaired bacterial killing by alveolar macrophages. Surprisingly, reduced neutrophil accumulation is likely not explained by reductions in phagocyte-recruiting chemokines or alterations in proinflammatory cytokine production compared with mice infected with S. aureus alone. Neutrophils from RSV-infected mice retain their ability to migrate toward chemokine signals, and neutrophils from the RSV-infected lung are better able to phagocytize and kill S. aureus ex vivo on a per cell basis. In contrast, while alveolar macrophages could ingest USA300 post-RSV, intracellular bacterial killing was impaired. The RSV/S. aureus coinfected lung promotes a state of overactivation in neutrophils, demonstrated by increased production of reactive oxygen species (ROS) that can drive formation of neutrophil extracellular traps (NETs), resulting in cell death. Mice with RSV/S. aureus coinfection had increased extracellular DNA and protein in bronchoalveolar lavage fluid and histological evidence confirmed NETosis in vivo. Taken together, these data highlight that prior RSV infection can prime the overactivation of neutrophils leading to cell death that impairs neutrophil accumulation in the lung. Additionally, alveolar macrophage killing of bacteria is impaired post-RSV. Together, these defects enhance USA300 MRSA bacterial growth in the lung post-RSV.
Keywords: MRSA; alveolar macrophage; lung; neutrophil; respiratory syncitial virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Liu Y, Ling L, Wong SH, Wang MH, Fitzgerald JR, Zou X, Fang S, Liu X, Wang X, Hu W, Chan H, Wang Y, Huang D, Li Q, Wong WT, Choi G, Zou H, Hui DS, Yu J, Tse G, Gin T, Wu WK, Chan MT, Zhang L. 2021. Outcomes of respiratory viral-bacterial co-infection in adult hospitalized patients. EClinicalMedicine 37:100955. doi:10.1016/j.eclinm.2021.100955 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F31 HL152509/HL/NHLBI NIH HHS/United States
- R35HL160770/HL/NHLBI NIH HHS/United States
- R35HL144481/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R35 HL160770/HL/NHLBI NIH HHS/United States
- R35 HL144481/HL/NHLBI NIH HHS/United States
- R35 HL150682/HL/NHLBI NIH HHS/United States
- R35HL150682/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- F31DK163865/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- University of Michigan Postdoctoral Pioneer Program
- T32 AI007413/AI/NIAID NIH HHS/United States
- T32AI007413/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
LinkOut - more resources
Full Text Sources
Medical

